Diabetes Metab J.
2013 Aug;37(4):286-290. 10.4093/dmj.2013.37.4.286.
Effect of Granulocyte Colony-Stimulating Factor on the Peripheral Nerves in Streptozotocin-Induced Diabetic Rat
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Research Institute of Clinical Medicine, Chonbuk National University Hospital, Chonbuk National University Medical School, Jeonju, Korea. pts@chonbuk.ac.kr
- 2Yeolin Hospital, Department of Internal Medicine, Jeonju, Korea.
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Research Institute of Clinical Medicine, Eulji University Hospital, Eulji University School of Medicine, Daejeon, Korea.
- KMID: 2174270
- DOI: http://doi.org/10.4093/dmj.2013.37.4.286
Abstract
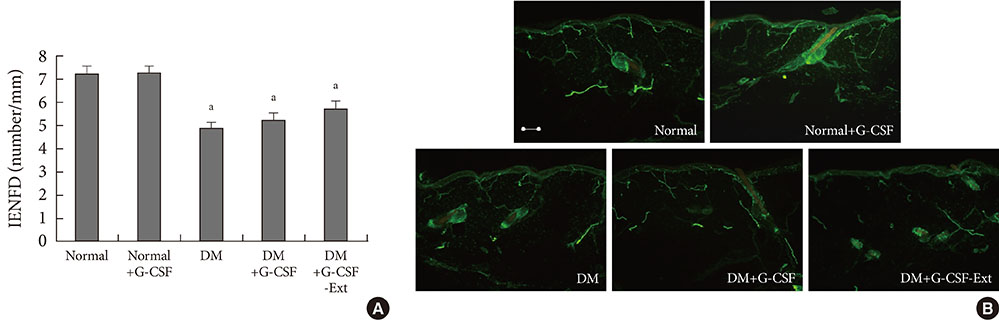
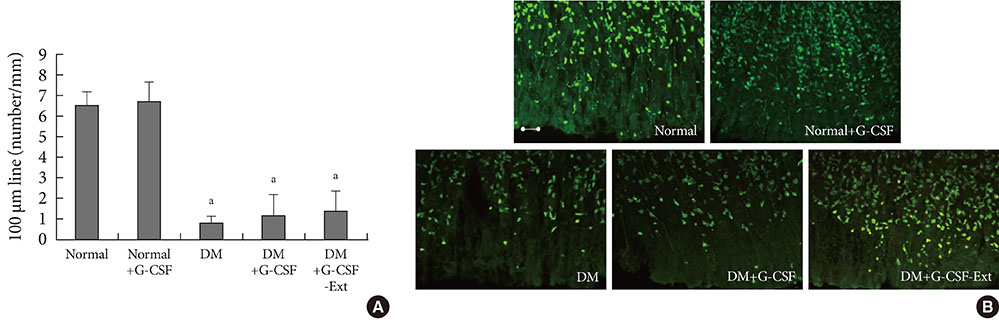
- There are controversial reports about the effect of granulocyte colony-stimulating factor (G-CSF) in peripheral nerve protection. Therefore, the present study aimed to investigate the effect of G-CSF on peripheral nerves in streptozotocin (STZ) induced diabetic rats. After STZ or vehicle injection, rats were divided into five groups (n=6) as follows: normal+vehicle, normal+G-CSF (50 microg/kg for 5 days), diabetes mellitus (DM)+vehicle, DM+G-CSF (50 microg/kg for 5 days), and DM+G-CSF extension (50 microg/kg for 5 days and followed by two injections per week up to 24 weeks). Our results showed that the current perception threshold was not significantly different among experimental groups. G-CSF treatment inhibited the loss of cutaneous nerves and gastric mucosal small nerve fibers in morphometric comparison, but statistical significance was not observed. The present results demonstrated that G-CSF has no harmful but minimal beneficial effects with respect to peripheral nerve preservation in diabetic rats.
MeSH Terms
Figure
Reference
-
1. Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991. 78:2791–2808.2. Pan HC, Cheng FC, Lai SZ, Yang DY, Wang YC, Lee MS. Enhanced regeneration in spinal cord injury by concomitant treatment with granulocyte colony-stimulating factor and neuronal stem cells. J Clin Neurosci. 2008. 15:656–664.3. Pan HC, Chen CJ, Cheng FC, Ho SP, Liu MJ, Hwang SM, Chang MH, Wang YC. Combination of G-CSF administration and human amniotic fluid mesenchymal stem cell transplantation promotes peripheral nerve regeneration. Neurochem Res. 2009. 34:518–527.4. Lu CZ, Xiao BG. G-CSF and neuroprotection: a therapeutic perspective in cerebral ischaemia. Biochem Soc Trans. 2006. 34(Pt 6):1327–1333.5. Meuer K, Pitzer C, Teismann P, Kruger C, Goricke B, Laage R, Lingor P, Peters K, Schlachetzki JC, Kobayashi K, Dietz GP, Weber D, Ferger B, Schabitz WR, Bach A, Schulz JB, Bahr M, Schneider A, Weishaupt JH. Granulocyte-colony stimulating factor is neuroprotective in a model of Parkinson's disease. J Neurochem. 2006. 97:675–686.6. Liou JT, Lui PW, Liu FC, Lai YS, Day YJ. Exogenous granulocyte colony-stimulating factor exacerbate pain-related behaviors after peripheral nerve injury. J Neuroimmunol. 2011. 232:83–93.7. Campbell IK, Rich MJ, Bischof RJ, Hamilton JA. The colony-stimulating factors and collagen-induced arthritis: exacerbation of disease by M-CSF and G-CSF and requirement for endogenous M-CSF. J Leukoc Biol. 2000. 68:144–150.8. Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003. 26:1790–1795.9. Cunha JM, Funez MI, Cunha FQ, Parada CA, Ferreira SH. Streptozotocin-induced mechanical hypernociception is not dependent on hyperglycemia. Braz J Med Biol Res. 2009. 42:197–206.10. Jin HY, Liu WJ, Park JH, Baek HS, Park TS. Effect of dipeptidyl peptidase-IV (DPP-IV) inhibitor (Vildagliptin) on peripheral nerves in streptozotocin-induced diabetic rats. Arch Med Res. 2009. 40:536–544.11. Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab. 2009. 94:2157–2163.12. Apfel SC. Neurotrophic factors and diabetic peripheral neuropathy. Eur Neurol. 1999. 41:Suppl 1. 27–34.13. Pan HC, Wu HT, Cheng FC, Chen CH, Sheu ML, Chen CJ. Potentiation of angiogenesis and regeneration by G-CSF after sciatic nerve crush injury. Biochem Biophys Res Commun. 2009. 382:177–182.14. Chao PK, Lu KT, Lee YL, Chen JC, Wang HL, Yang YL, Cheng MY, Liao MF, Ro LS. Early systemic granulocyte-colony stimulating factor treatment attenuates neuropathic pain after peripheral nerve injury. PLoS One. 2012. 7:e43680.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of granulocyte colony-stimulating factor in chemotherapy of acute myelogenous leukemia
- Sweet Syndrome in a Child with Aplastic Anemia after Receiving Recombinant Granulocyte Colony-stimulating Factor
- Two cases of congenital agranulocytosis treated with recombinant human granulocyte colony-stimulating factor
- Effect of combination gene therapy with herpes simplex virus thymidine kinase suicidal gene and granulocyte-macrophage colony-stimulating factor gene in murine melanoma model
- The effects on the production of platelet activating factor in the cultured human endothelial cells by interleukin-6 and granulocyte macrophage colony stimulating factor