Ann Dermatol.
2008 Jun;20(2):49-55. 10.5021/ad.2008.20.2.49.
COMP-angiopoietin 1 Gene Transfer Enhances Cutaneous Wound Healing by Promoting Angiogenesis
- Affiliations
-
- 1Department of Dermatology, Kangnam St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Korea. yymmpark@hotmail.com
- 2Gowoonsesang Dermatology Clinic, Korea.
- 3Department of Neurology, Kangnam St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 4Department of Biological Sciences, Biomedical Center, Korea Advanced Institute of Science and Technology, Daejeon, Korea.
- KMID: 2172084
- DOI: http://doi.org/10.5021/ad.2008.20.2.49
Abstract
-
BACKGROUND: Angiogenesis is crucial for wound healing and exogenous supplements of the angiogenic growth factors have been known to promote cutaneous wound healing. Angiopoietin (Ang) 1 is a recently discovered angiogenic factor and there have been few studies of its effect on cutaneous wound healing.
OBJECTIVE
We examined the effect of Ang 1 on cutaneous wound healing.
METHODS
Cartilage oligomeric matrix protein (COMP)-Ang 1 (Ade-COMP-Ang 1)- was intravenously injected to rats two days before surgery creating full-thickness wounds. The clinical wound healing rate and the number of vessels in the skin samples were evaluated on days 3, 7 and 14 post operation.
RESULTS
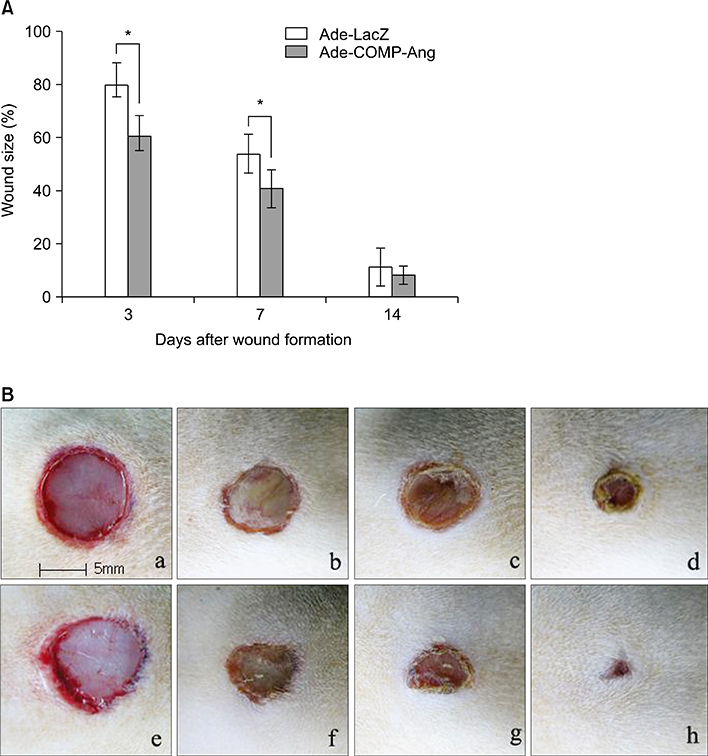
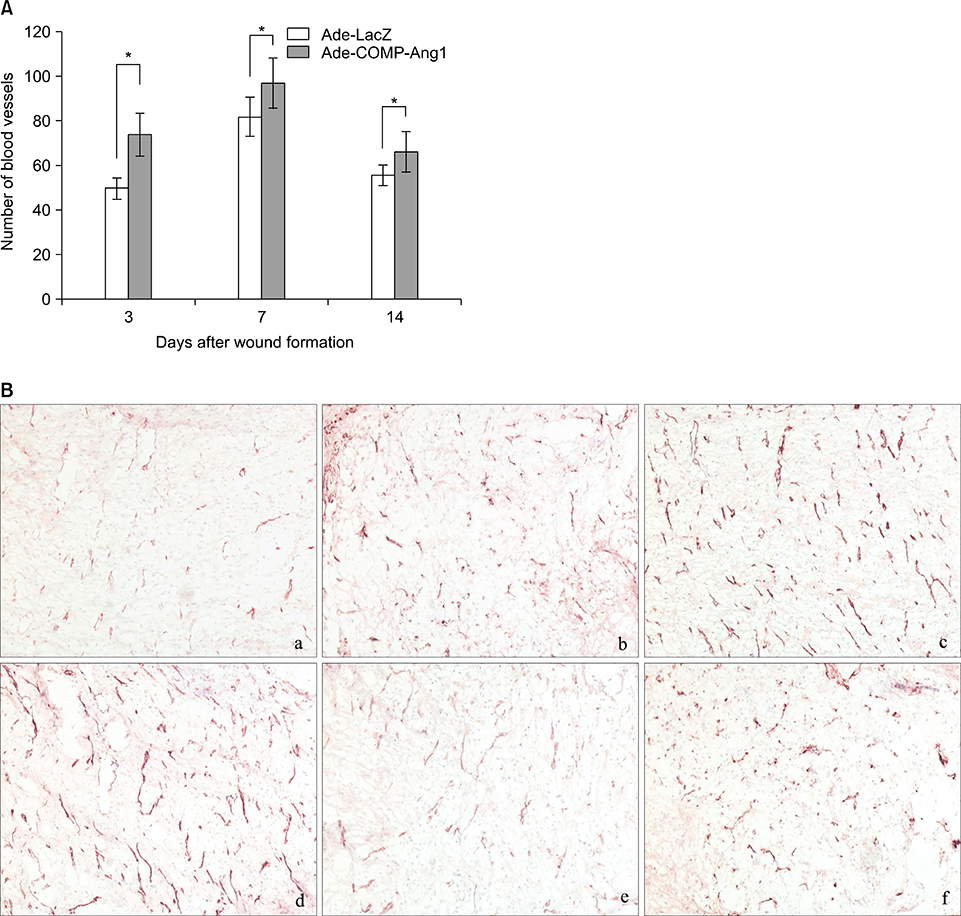
At post-operation day 3, 7 and 14, the clinical wound healing rate was 38.3%, 59.4% and 92.1%, respectively, in the Ade-COMP-Ang 1-treated group, compared with 20.5%, 47.5% and 87.3%, respectively, in the Ade-LacZ-treated group. There were significant differences in the results of day 3 and day 7 between two groups (p<0.05). Histopathologically, the number of the vessels of the Ade-COMP-Ang 1-treated group was 73.7, 94.1 and 62.7 at day 3, 7 and 14, compared with that of the Ade-LacZ-treated group, 53.5, 83.9, and 56.9. The differences in the results of the two groups were statistically significant (p<0.05).
CONCLUSION
These results indicate that Ade-COMP-Ang 1 therapy significantly accelerats wound healing by promoting angiogenesis. However, further study using Ade-COMP-Ang 1 gene therapy for chronic wounds in which the formation of new blood vessels is impaired is needed in the near future.
MeSH Terms
Figure
Reference
-
1. Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000; 407:242–248.
Article2. Kämpfer H, Pfeilschifter J, Frank S. Expressional regulation of angiopoietin-1 and 2 and the Tie-1 and 2 receptor tyrosine kinases during cutaneous wound healing: A comparative study of normal and impaired repair. Lab Invest. 2001; 81:361–373.
Article3. Colwell AS, Beanes SR, Soo C, Dang C, Ting K, Longaker MT, et al. Increased angiogenesis and expression of vascular endothelial growth factor during scarless repair. Plast Reconstr Surg. 2005; 115:204–212.4. Yao F, Visovatti S, Johnson CS, Chen M, Slama J, Wenger A, et al. Age and growth factors in porcine full-thickness wound healing. Wound Repair Regen. 2001; 9:371–377.
Article5. Drinkwater SL, Burnard KG, Ding R, Smith A. Increased but ineffectual angiogenic drive in nonhealing venous leg ulcers. J Vasc Surg. 2003; 38:1106–1112.
Article6. Romano Di Peppe S, Mangoni A, Zambruno G, Spinetti G, Melillo G, Napolitano M, et al. Adenovirus- mediated VEGF165 gene transfer enhances wound healing by promoting angiogenesis in CD1 diabetic mice. Gene Therapy. 2002; 9:1271–1277.
Article7. Galeano M, Altavilla D, Cucinotta D, Russo GT, Calò M, Bitto A, et al. Recombinant human erythropoietin stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetes. 2004; 53:2509–2517.
Article8. Cianfarani F, Tommasi R, Failla CM, Viviano MT, Annessi G, Papi M, et al. Granulocyte/macrophage colony-stimulating factor treatment of human chronic ulcers promotes angiogenesis associated with de novo vascular endothelial growth factor transcription in the ulcer bed. Br J Dermatol. 2006; 154:34–41.
Article9. Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996; 87:1161–1169.
Article10. Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, et al. COMP-Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. PNAS. 2004; 101:5547–5552.
Article11. Cho CH, Kammerer RA, Lee HJ, Yasunaga K, Kim KT, Choi HH, et al. Designed angiopoietin-1 variant, COMP-Ang1, protects against radiation-induced endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004; 101:5553–5558.
Article12. Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996; 87:1171–1180.
Article13. Sato TN, Qin Y, Kozak CA, Audus KL. Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci U S A. 1993; 90:9355–9358.
Article14. Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, et al. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998; 282:468–471.
Article15. Jones MK, Kawanaka H, Baatar D, Szabó IL, Tsugawa K, Pai R, et al. Gene therapy for gastric ulcers with single local injection of naked DNA encoding VEGF and angiopoietin-1. Gastroenterology. 2001; 121:1040–1047.
Article16. Jung H, Gurunluoglu R, Scharpf J, Siemionow M. Adenovirus-mediated angiopoietin-1 gene therapy enhances skin flap survival. Microsurgery. 2003; 23:374–380.
Article17. Kim GM, Lee WS, Hwang SJ, Kye YC, Kim HO, Kim SY. The study of wound healing using cultured autologous dermal fibroblast of guinea pig. Korean J Dermatol. 2005; 43:576–586.18. Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000; 103:898–901.19. Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000; 6:460–463.
Article20. Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999; 286:2511–2514.
Article21. Baffert F, Le T, Thurston G, McDonald DM. Angiopoietin-1 decreases plasma leakage by reducing number and size of endothelial gaps in venules. Am J Physiol Heart Circ Physiol. 2006; 290:H107–H118.
Article22. Jho D, Mehta D, Ahmmed G, Gao XP, Tiruppathi C, Broman M, et al. Angiopoietin-1 opposes VEGF-induced increase in endothelial permeability by inhibiting TRPC1-dependent Ca2+ influx. CircRes. 2005; 96:1282–1290.
Article23. Galeano M, Deodato B, Altavilla D, Cucinotta D, Arsic N, Marini H, et al. Adeno-associated viral vector-mediated human vascular endothelial growth factor gene transfer stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetologia. 2003; 46:546–555.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- COMP-Angiopoietin-1 Promotes Cavernous Angiogenesis in a Type 2 Diabetic Rat Model
- Improvement of the Inferior Epigastric Artery Flap Viability Using Adenovirus-mediated VEGF and COMP-angiopoietin-1
- Angiopoietin-1 Gene Therapy Attenuates Hypertension and Target Organ Damage in Nitric Oxide Synthase Inhibited Spontaneously Hypertensive Rats
- Fabricating Composite Cell Sheets for Wound Healing: Cell Sheets Based on the Communication Between BMSCs and HFSCs Facilitate Full-Thickness Cutaneous Wound Healing
- Gene therapy of scarring: a lesson learned from fetal scarless wound healing