Anat Cell Biol.
2010 Jun;43(2):140-149. 10.5115/acb.2010.43.2.140.
Systemic injection of recombinant human erythropoietin after focal cerebral ischemia enhances oligodendroglial and endothelial progenitor cells in rat brain
- Affiliations
-
- 1Department of Laboratory Medicine, Masansamsung Medical Center, School of Medicine, Sungkyunkwan University, Masan, Korea.
- 2Department of Anatomy, College of Medicine, Dongguk University, Gyeongju, Korea. jungyw@dongguk.ac.kr
- KMID: 2168887
- DOI: http://doi.org/10.5115/acb.2010.43.2.140
Abstract
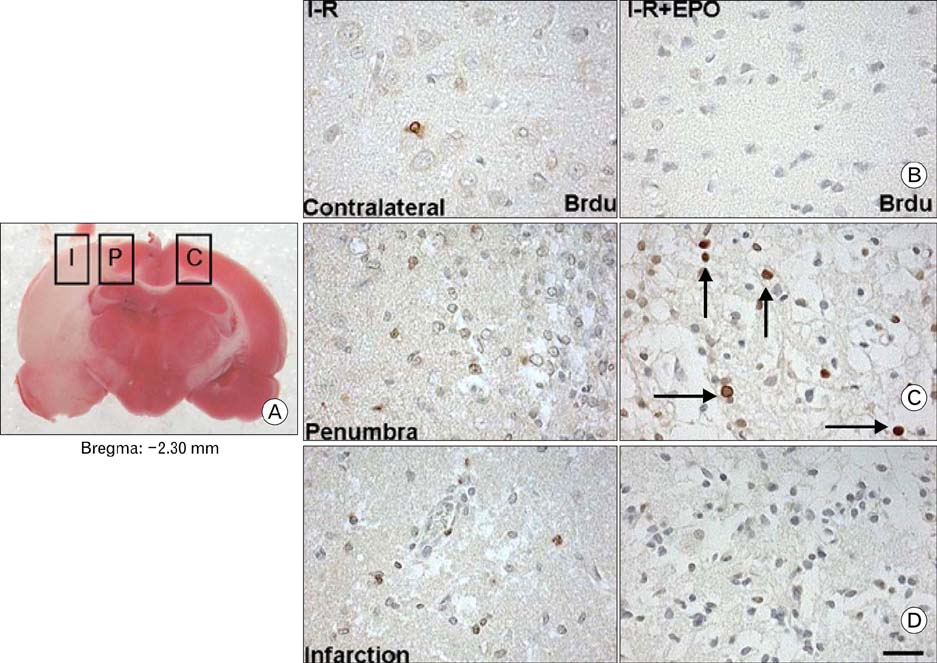
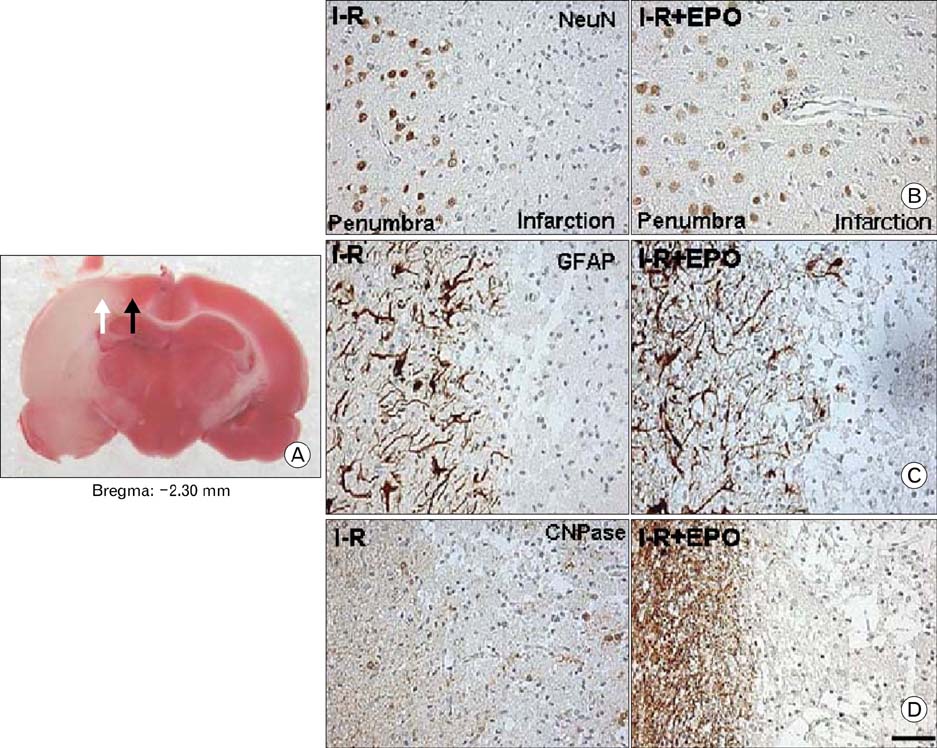
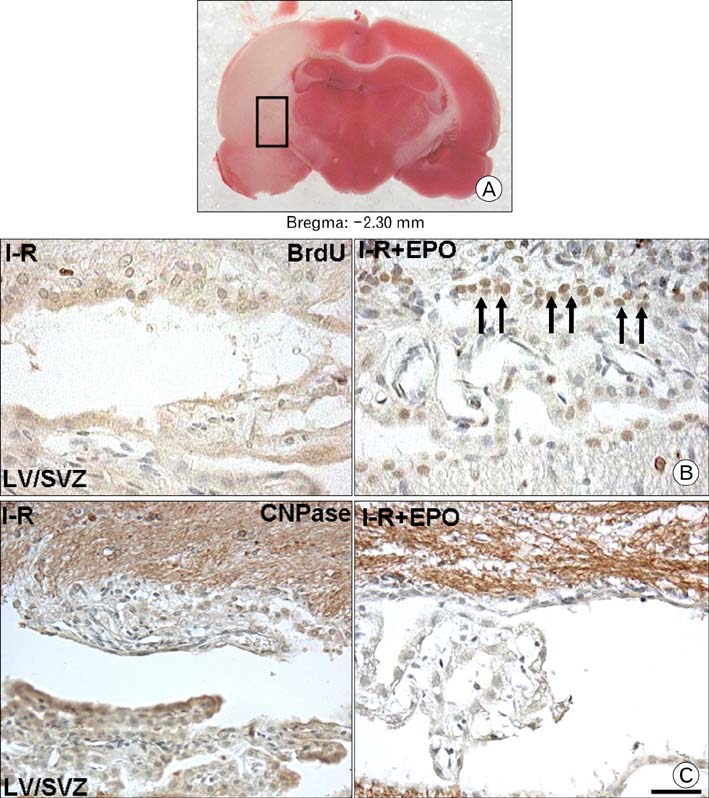
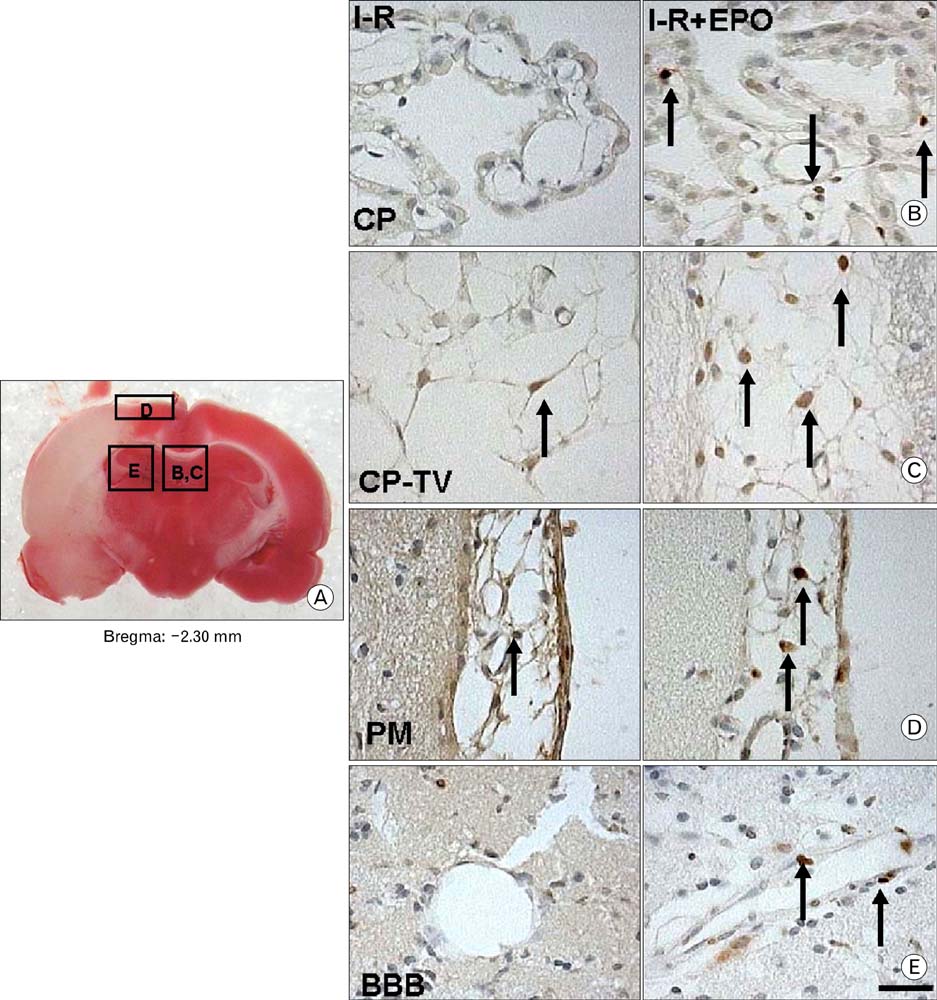
- Erythropoietin (EPO) has been demonstrated the ability of recombinant human erythropoietin (r-Hu-EPO), when administered intracerebro-ventricularly, to improve stroke outcome through the reduction of stroke damage. In a brain ischemic model, however, systemic administration of r-Hu-EPO has not been intensely investigated given that in general, large glycosylated molecules have been deemed incapable of crossing the blood-brain barrier. In this study, administration of r-Hu-EPO for 4 days, intraperitoneally after ischemia-reperfusion (I-R) increased the number of bromodeoxyuridine (BrdU)-positive cells in the penumbra (10.1+/-1.4, n=5, P<0.05) and in the subventricular zone (SVZ) of the lateral ventricle (LV) (25+/-2.7, n=5, P<0.05) as compared with those of I-R (penumbra: 2.5+/-0.7; SVZ of LV: 3.8+/-1.5). A significant increase of BrdU-positive cells in these areas was coincident with a strong immunoreactivity of oligodendrocyte progenitor cell marker (2', 3'-cyclic nucleotide 3'-phosphodiesterase). Furthermore, r-Hu-EPO administration increased the number of BrdU-positive cells in the choroid plexus (7.8+/-2.3, n=5, P<0.05) and in cerebral blood vessels (3.5+/-1.3, n=5, P<0.05) when compared with those of I-R (choroid plexus: 1.2+/-0.5; cerebral blood vessels: 0.6+/-0.1). These results suggest that, even when systemically administered, r-Hu-EPO may have therapeutic potential for stroke via the proliferation of oligodendroglial and endothelial progenitor cells.
MeSH Terms
Figure
Reference
-
1. Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002. 8:963–970.2. Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999. 85:221–228.3. Bernaudin M, Marti HH, Roussel S, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999. 19:643–651.4. Brines ML, Ghezzi P, Keenan S, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000. 97:10526–10531.5. Bu J, Banki A, Wu Q, Nishiyama A. Increased NG2+ glial cell proliferation and oligodendrocyte generation in the hypomyelinating mutant shiverer. Glia. 2004. 48:51–63.6. Calvillo L, Latini R, Kajstura J, et al. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc Natl Acad Sci U S A. 2003. 100:4802–4806.7. Carlini RG, Reyes AA, Rothstein M. Recombinant human erythropoietin stimulates angiogenesis in vitro. Kidney Int. 1995. 47:740–745.8. Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002. 106:2973–2979.9. Crawford LE, Milliken EE, Irani K, et al. Superoxide-mediated actin response in post-hypoxic endothelial cells. J Biol Chem. 1996. 271:26863–26867.10. Digicaylioglu M, Bichet S, Marti HH, et al. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc Natl Acad Sci U S A. 1995. 92:3717–3720.11. Egrie JC, Strickland TW, Lane J, et al. Characterization and biological effects of recombinant human erythropoietin. Immunobiology. 1986. 172:213–224.12. Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998. 36:249–266.13. Girard C, Bemelmans AP, Dufour N, et al. Grafts of brain-derived neurotrophic factor and neurotrophin 3-transduced primate Schwann cells lead to functional recovery of the demyelinated mouse spinal cord. J Neurosci. 2005. 25:7924–7933.14. Gottlieb M, Domercq M, Matute C. Altered expression of the glutamate transporter EAAC1 in neurons and immature oligodendrocytes after transient forebrain ischemia. J Cereb Blood Flow Metab. 2000. 20:678–687.15. Grasso G, Buemi M, Alafaci C, et al. Beneficial effects of systemic administration of recombinant human erythropoietin in rabbits subjected to subarachnoid hemorrhage. Proc Natl Acad Sci U S A. 2002. 99:5627–5631.16. Hasegawa H, Ma T, Skach W, Matthay MA, Verkman AS. Molecular cloning of a mercurial-insensitive water channel expressed in selected water-transporting tissues. J Biol Chem. 1994. 269:5497–5500.17. Kadota T, Shingo T, Yasuhara T, et al. Continuous intraventricular infusion of erythropoietin exerts neuroprotective/rescue effects upon Parkinson's disease model of rats with enhanced neurogenesis. Brain Res. 2009. 1254:120–127.18. Kim HM, Hwang DH, Lee JE, Kim SU, Kim BG. Ex vivo VEGF delivery by neural stem cells enhances proliferation of glial progenitors, angiogenesis, and tissue sparing after spinal cord injury. PLoS One. 2009. 4:e4987.19. Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996. 16:2027–2033.20. Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999. 13:450–464.21. Levison SW, Rothstein RP, Romanko MJ, Snyder MJ, Meyers RL, Vannucci SJ. Hypoxia/ischemia depletes the rat perinatal subventricular zone of oligodendrocyte progenitors and neural stem cells. Dev Neurosci. 2001. 23:234–247.22. Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998. 18:7768–7778.23. Marti HH, Bernaudin M, Petit E, Bauer C. Neuroprotection and Angiogenesis: Dual Role of Erythropoietin in Brain Ischemia. News Physiol Sci. 2000. 15:225–229.24. Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997. 76:105–116.25. Moritz KM, Lim GB, Wintour EM. Developmental regulation of erythropoietin and erythropoiesis. Am J Physiol. 1997. 273:R1829–R1844.26. Ness JK, Valentino M, McIver SR, Goldberg MP. Identification of oligodendrocytes in experimental disease models. Glia. 2005. 50:321–328.27. Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000. 425:479–494.28. Pardridge WM. Drug delivery to the brain. J Cereb Blood Flow Metab. 1997. 17:713–731.29. Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002. 52:802–813.30. Pincus DW, Keyoung HM, Harrison-Restelli C, et al. Fibroblast growth factor-2/brain-derived neurotrophic factor-associated maturation of new neurons generated from adult human subependymal cells. Ann Neurol. 1998. 43:576–585.31. Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990. 110:1001–1020.32. Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis. 2004. 15:415–436.33. Ribatti D, Presta M, Vacca A, et al. Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood. 1999. 93:2627–2636.34. Sakanaka M, Wen TC, Matsuda S, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A. 1998. 95:4635–4640.35. Shi J, Marinovich A, Barres BA. Purification and characterization of adult oligodendrocyte precursor cells from the rat optic nerve. J Neurosci. 1998. 18:4627–4636.36. Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001. 21:9733–9743.37. Sirén AL, Fratelli M, Brines M, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001. 98:4044–4049.38. Skoff RP, Bessert DA, Barks JD, Song D, Cerghet M, Silverstein FS. Hypoxic-ischemic injury results in acute disruption of myelin gene expression and death of oligodendroglial precursors in neonatal mice. Int J Dev Neurosci. 2001. 19:197–208.39. Sugawa M, Sakurai Y, Ishikawa-Ieda Y, Suzuki H, Asou H. Effects of erythropoietin on glial cell development; oligodendrocyte maturation and astrocyte proliferation. Neurosci Res. 2002. 44:391–403.40. Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999. 5:434–438.41. Vitellaro-Zuccarello L, Mazzetti S, Madaschi L, et al. Chronic erythropoietin-mediated effects on the expression of astrocyte markers in a rat model of contusive spinal cord injury. Neuroscience. 2008. 151:452–466.42. Vogel V, Kramer HJ, Bäcker A, Meyer-Lehnert H, Jelkmann W, Fandrey J. Effects of erythropoietin on endothelin-1 synthesis and the cellular calcium messenger system in vascular endothelial cells. Am J Hypertens. 1997. 10:289–296.43. Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004. 35:1732–1737.44. Weiss S, Reynolds BA, Vescovi AL, Morshead C, Craig CG, van der Kooy D. Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. 1996. 19:387–393.45. Yan Y, Dempsey RJ, Sun D. Na+-K+-Cl- cotransporter in rat focal cerebral ischemia. J Cereb Blood Flow Metab. 2001. 21:711–721.46. Zhang F, Signore AP, Zhou Z, Wang S, Cao G, Chen J. Erythropoietin protects CA1 neurons against global cerebral ischemia in rat: potential signaling mechanisms. J Neurosci Res. 2006. 83:1241–1251.47. Zhang J, Li Y, Cui Y, et al. Erythropoietin treatment improves neurological functional recovery in EAE mice. Brain Res. 2005. 1034:34–39.48. Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000. 106:829–838.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Angiogenesis and cell therapy
- Animal Model of Cerebral Ischemia
- A case of acute myeloid leukemia in chronic renal failure patient on erythropoietin treatment
- The Effect of Erythropoietin on Ischemia-Reperfusion Injury: An Experimental Study in Rat TRAM Flap Model
- Changes in Evoked Potentials in Focal Cerebral Cortical Ischemia in the Rat