Anat Cell Biol.
2014 Mar;47(1):1-11. 10.5115/acb.2014.47.1.1.
The expressions of stem cell markers: Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Tcl1, Tbx3, Dppa4, and Esrrb in bladder, colon, and prostate cancer, and certain cancer cell lines
- Affiliations
-
- 1Department of Biology, Sanandaj Branch, Islamic Azad University, Sanandaj, Iran.
- 2Cellular and Molecular Research Center, Kurdistan University of Medical Sciences, Sanandaj, Iran. farfath@gmail.com
- 3Department of Surgery, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran. tayyeb.ghadimi5@gmail.com
- 4Department of Urology, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran.
- KMID: 2168851
- DOI: http://doi.org/10.5115/acb.2014.47.1.1
Abstract
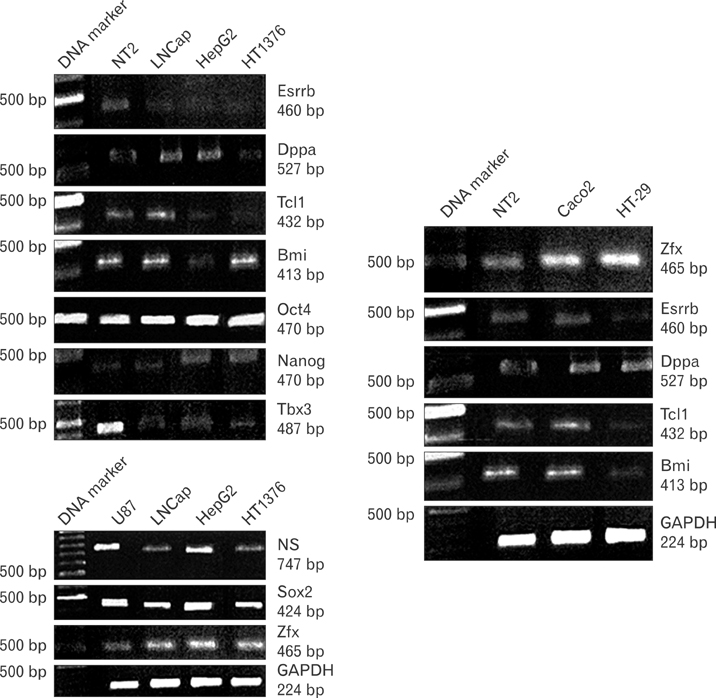
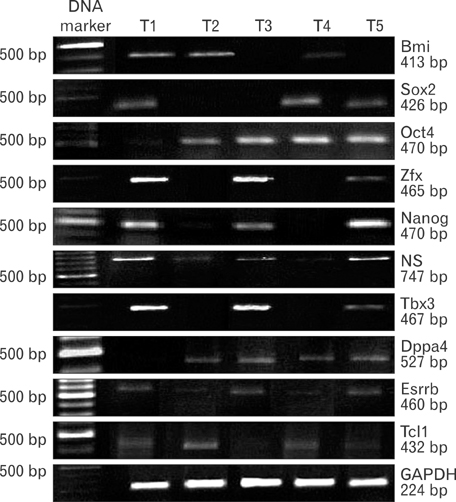
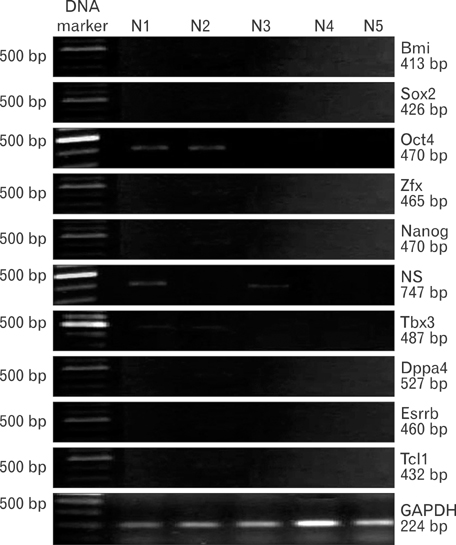
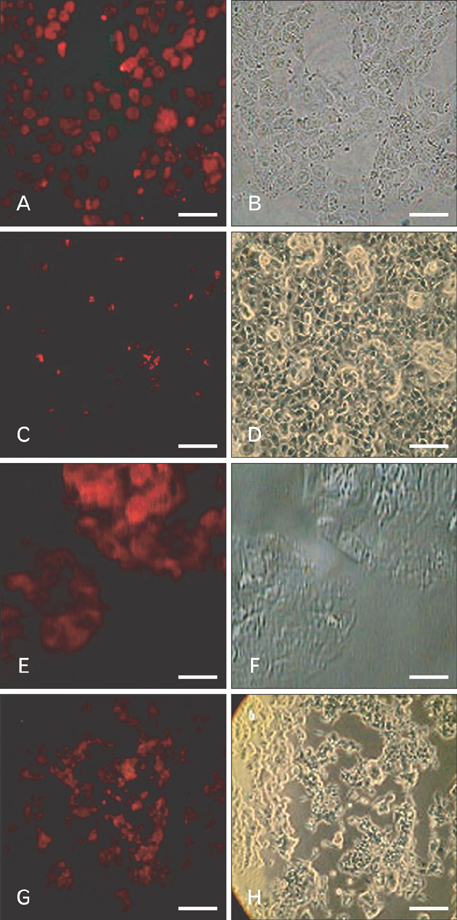
- Uncontrolled self-renewal plays a direct function in the progression of different types of carcinomas. The same molecular pathway that manages self-renewal in normal stem cells also seems to manage cancer stem cells. Here, we examine the expressions of self-renewal regulatory factors Oct4, Nanog, Sox2, nucleostemin, Zfx, Esrrb, Tcl1, Tbx3, and Dppa4 in tissue samples of colon, prostate, and bladder carcinomas as well as cancer cell lines HT-29, Caco-2, HT-1376, LNCaP, and HepG2. We used reverse transcriptase polymerase chain reaction to examine expressions of the above mentioned regulatory factors in cancer cell lines HT-29, Caco-2, HT-1376, LNCaP, and HepG2 and in 20 tumor tissue samples. Total RNA was isolated by the ISOGEN method. RNA integrity was checked by agarose gel electrophoresis and spectrophotometry. Expressions of Oct4 and nucleostemin at the protein level were determined by immunocytochemistry. A significant relationship was found between tumor grade and self-renewal gene expression. Expressions of stem cell specific marker genes were detected in all examined cancer cell lines, in 40% to 100% of bladder cancer samples, and in 60% to 100% of colon and prostate cancer samples. Oct4 expressed in 100% of tumor tissue samples. Our data show that stem cell markers Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Esrrb, Tcl1, Tbx3, and Dppa4 significantly express in cancer cell lines and cancer tissues. Hence, these markers might be useful as potential tumor markers in the diagnosis and/or prognosis of tumors.
Keyword
MeSH Terms
-
Cell Line*
Colon*
Colonic Neoplasms
Diagnosis
Electrophoresis, Agar Gel
Gene Expression
Immunohistochemistry
Neoplastic Stem Cells
Prognosis
Prostate*
Prostatic Neoplasms*
Reverse Transcriptase Polymerase Chain Reaction
RNA
Spectrophotometry
Stem Cells*
Biomarkers, Tumor
Urinary Bladder Neoplasms
Urinary Bladder*
RNA
Figure
Reference
-
1. Cho RW, Clarke MF. Recent advances in cancer stem cells. Curr Opin Genet Dev. 2008; 18:48–53.2. Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006; 124:1111–1115.3. Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004; 23:7274–7282.4. Klonisch T, Wiechec E, Hombach-Klonisch S, Ande SR, Wesselborg S, Schulze-Osthoff K, Los M. Cancer stem cell markers in common cancers: therapeutic implications. Trends Mol Med. 2008; 14:450–460.5. Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003; 3:895–902.6. Lee J, Kim HK, Rho JY, Han YM, Kim J. The human OCT-4 isoforms differ in their ability to confer self-renewal. J Biol Chem. 2006; 281:33554–33565.7. Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005; 26:495–502.8. Bani-Yaghoub M, Tremblay RG, Lei JX, Zhang D, Zurakowski B, Sandhu JK, Smith B, Ribecco-Lutkiewicz M, Kennedy J, Walker PR, Sikorska M. Role of Sox2 in the development of the mouse neocortex. Dev Biol. 2006; 295:52–66.9. Schoenhals M, Kassambara A, De Vos J, Hose D, Moreaux J, Klein B. Embryonic stem cell markers expression in cancers. Biochem Biophys Res Commun. 2009; 383:157–162.10. Pan G, Thomson JA. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 2007; 17:42–49.11. Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003; 113:643–655.12. Ezeh UI, Turek PJ, Reijo RA, Clark AT. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer. 2005; 104:2255–2265.13. Fan Y, Liu Z, Zhao S, Lou F, Nilsson S, Ekman P, Xu D, Fang X. Nucleostemin mRNA is expressed in both normal and malignant renal tissues. Br J Cancer. 2006; 94:1658–1662.14. Kafienah W, Mistry S, Williams C, Hollander AP. Nucleostemin is a marker of proliferating stromal stem cells in adult human bone marrow. Stem Cells. 2006; 24:1113–1120.15. Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003; 423:255–260.16. Leung C, Lingbeek M, Shakhova O, Liu J, Tanger E, Saremaslani P, Van Lohuizen M, Marino S. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature. 2004; 428:337–341.17. Qin ZK, Yang JA, Ye YL, Zhang X, Xu LH, Zhou FJ, Han H, Liu ZW, Song LB, Zeng MS. Expression of Bmi-1 is a prognostic marker in bladder cancer. BMC Cancer. 2009; 9:61.18. Cellot S, Sauvageau G. Zfx: at the crossroads of survival and self-renewal. Cell. 2007; 129:239–241.19. Galan-Caridad JM, Harel S, Arenzana TL, Hou ZE, Doetsch FK, Mirny LA, Reizis B. Zfx controls the self-renewal of embryonic and hematopoietic stem cells. Cell. 2007; 129:345–357.20. Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, DeCoste C, Schafer X, Lun Y, Lemischka IR. Dissecting self-renewal in stem cells with RNA interference. Nature. 2006; 442:533–538.21. Lock RB. TCL1: a new drug target in lymphoid and germ-cell malignancies? Int J Biochem Cell Biol. 2003; 35:1614–1618.22. Zhang X, Zhang J, Wang T, Esteban MA, Pei D. Esrrb activates Oct4 transcription and sustains self-renewal and pluripotency in embryonic stem cells. J Biol Chem. 2008; 283:35825–35833.23. Chakravarthy H, Boer B, Desler M, Mallanna SK, McKeithan TW, Rizzino A. Identification of DPPA4 and other genes as putative Sox2:Oct-3/4 target genes using a combination of in silico analysis and transcription-based assays. J Cell Physiol. 2008; 216:651–662.24. Tejpar S. The multidisciplinary management of gastrointestinal cancer. The use of molecular markers in the diagnosis and treatment of colorectal cancer. Best Pract Res Clin Gastroenterol. 2007; 21:1071–1087.25. Cohen SM, Shirai T, Steineck G. Epidemiology and etiology of premalignant and malignant urothelial changes. Scand J Urol Nephrol Suppl. 2000; (205):105–115.26. Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999; 49:33–64.27. Kermani AJ, Fathi F, Mowla SJ. Characterization and genetic manipulation of human umbilical cord vein mesenchymal stem cells: potential application in cell-based gene therapy. Rejuvenation Res. 2008; 11:379–386.28. Matin MM, Walsh JR, Gokhale PJ, Draper JS, Bahrami AR, Morton I, Moore HD, Andrews PW. Specific knockdown of Oct4 and beta2-microglobulin expression by RNA interference in human embryonic stem cells and embryonic carcinoma cells. Stem Cells. 2004; 22:659–668.29. Moserle L, Ghisi M, Amadori A, Indraccolo S. Side population and cancer stem cells: therapeutic implications. Cancer Lett. 2010; 288:1–9.30. Schulz WA, Hoffmann MJ. Transcription factor networks in embryonic stem cells and testicular cancer and the definition of epigenetics. Epigenetics. 2007; 2:37–42.31. Santagata S, Ligon KL, Hornick JL. Embryonic stem cell transcription factor signatures in the diagnosis of primary and metastatic germ cell tumors. Am J Surg Pathol. 2007; 31:836–845.32. Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005; 121:465–477.33. Atlasi Y, Mowla SJ, Ziaee SA, Bahrami AR. OCT-4, an embryonic stem cell marker, is highly expressed in bladder cancer. Int J Cancer. 2007; 120:1598–1602.34. Jin T, Branch DR, Zhang X, Qi S, Youngson B, Goss PE. Examination of POU homeobox gene expression in human breast cancer cells. Int J Cancer. 1999; 81:104–112.35. Looijenga LH, Stoop H, de Leeuw HP, de Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, van Zoelen EJ, Weber RF, Wolffenbuttel KP, van Dekken H, Honecker F, Bokemeyer C, Perlman EJ, Schneider DT, Kononen J, Sauter G, Oosterhuis JW. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 2003; 63:2244–2250.36. Amini S, Fathi F, Parivar K, Kuchesfahani HM, Rezaie MJ, Nikkhoo B. Evaluating the expression of Oct4, NANOG, Sox2 and nucleostemin in colon cancer cell lines (Caco-2 and HT-29). Yakhteh Med J. 2010; 12:223–230.37. Matthai C, Horvat R, Noe M, Nagele F, Radjabi A, van Trotsenburg M, Huber J, Kolbus A. Oct-4 expression in human endometrium. Mol Hum Reprod. 2006; 12:7–10.38. Hoei-Hansen CE, Kraggerud SM, Abeler VM, Kaern J, Rajpert-De Meyts E, Lothe RA. Ovarian dysgerminomas are characterised by frequent KIT mutations and abundant expression of pluripotency markers. Mol Cancer. 2007; 6:12.39. Cheng L, Sung MT, Cossu-Rocca P, Jones TD, MacLennan GT, De Jong J, Lopez-Beltran A, Montironi R, Looijenga LH. OCT4: biological functions and clinical applications as a marker of germ cell neoplasia. J Pathol. 2007; 211:1–9.40. Tsai RY, McKay RD. A nucleolar mechanism controlling cell proliferation in stem cells and cancer cells. Genes Dev. 2002; 16:2991–3003.41. Tsai RY, McKay RD. A multistep, GTP-driven mechanism controlling the dynamic cycling of nucleostemin. J Cell Biol. 2005; 168:179–184.42. Chen JC, Goldhamer DJ. Skeletal muscle stem cells. Reprod Biol Endocrinol. 2003; 1:101.43. Berendse M, Grounds MD, Lloyd CM. Myoblast structure affects subsequent skeletal myotube morphology and sarcomere assembly. Exp Cell Res. 2003; 291:435–450.44. Liu SJ, Cai ZW, Liu YJ, Dong MY, Sun LQ, Hu GF, Wei YY, Lao WD. Role of nucleostemin in growth regulation of gastric cancer, liver cancer and other malignancies. World J Gastroenterol. 2004; 10:1246–1249.45. Liu RL, Zhang ZH, Zhao WM, Wang M, Qi SY, Li J, Zhang Y, Li SZ, Xu Y. Expression of nucleostemin in prostate cancer and its effect on the proliferation of PC-3 cells. Chin Med J (Engl). 2008; 121:299–304.46. Yang HX, Jin GL, Meng L, Zhang JZ, Liu WB, Shou CC. Screening and identification of proteins interacting with nucleostemin. World J Gastroenterol. 2005; 11:4812–4814.47. Zhou Y, Su Z, Huang Y, Sun T, Chen S, Wu T, Chen G, Xie X, Li B, Du Z. The Zfx gene is expressed in human gliomas and is important in the proliferation and apoptosis of the human malignant glioma cell line U251. J Exp Clin Cancer Res. 2011; 30:114.48. Lau SK, Weiss LM, Chu PG. TCL1 protein expression in testicular germ cell tumors. Am J Clin Pathol. 2010; 133:762–766.49. Ito A, Asamoto M, Hokaiwado N, Takahashi S, Shirai T. Tbx3 expression is related to apoptosis and cell proliferation in rat bladder both hyperplastic epithelial cells and carcinoma cells. Cancer Lett. 2005; 219:105–112.50. Yarosh W, Barrientos T, Esmailpour T, Lin L, Carpenter PM, Osann K, Anton-Culver H, Huang T. TBX3 is overexpressed in breast cancer and represses p14 ARF by interacting with histone deacetylases. Cancer Res. 2008; 68:693–699.51. Renard CA, Labalette C, Armengol C, Cougot D, Wei Y, Cairo S, Pineau P, Neuveut C, de Reyniès A, Dejean A, Perret C, Buendia MA. Tbx3 is a downstream target of the Wnt/betacate nin pathway and a critical mediator of beta-catenin survival functions in liver cancer. Cancer Res. 2007; 67:901–910.52. Lomnytska M, Dubrovska A, Hellman U, Volodko N, Souchelnytskyi S. Increased expression of cSHMT, Tbx3 and utrophin in plasma of ovarian and breast cancer patients. Int J Cancer. 2006; 118:412–421.53. van den Berg DL, Zhang W, Yates A, Engelen E, Takacs K, Bezstarosti K, Demmers J, Chambers I, Poot RA. Estrogen-related receptor beta interacts with Oct4 to positively regulate Nanog gene expression. Mol Cell Biol. 2008; 28:5986–5995.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation of Cancer Stem-Cell Markers OCT4, SOX2, and NANOG with Clinicopathological Features and Prognosis in Operative Patients with Rectal Cancer
- Metastasis prognostic factors and cancer stem cell-related transcription factors associated with metastasis induction in canine metastatic mammary gland tumors
- The Role and Specific Mechanism of OCT4 in Cancer Stem Cells: A Review
- OCT4 Expression Enhances Features of Cancer Stem Cells in a Mouse Model of Breast Cancer
- Enhanced proliferation and differentiation of Oct4- and Sox2-overexpressing human adipose tissue mesenchymal stem cells