Yonsei Med J.
2018 Jan;59(1):35-42. 10.3349/ymj.2018.59.1.35.
Correlation of Cancer Stem-Cell Markers OCT4, SOX2, and NANOG with Clinicopathological Features and Prognosis in Operative Patients with Rectal Cancer
- Affiliations
-
- 1Department of General Surgery, The Second Affiliated Hospital of Harbin Medical University, Harbin, China. huangyuenan@outlook.com
- 2Department of General Surgery, The First Affiliated Hospital of Harbin Medical University, Harbin, China.
- KMID: 2418846
- DOI: http://doi.org/10.3349/ymj.2018.59.1.35
Abstract
- PURPOSE
To investigate the association of cancer stem-cell markers [octamer-binding transcription factor 4 (OCT4), sex determining region Y-box 2 (SOX2), and Nanog homebox (NANOG)] expression with clinicopathological properties and overall survival (OS) in operative rectal cancer (RC) patients receiving adjuvant therapy.
MATERIALS AND METHODS
153 patients with primary RC receiving surgery were enrolled. Tumor tissue and paired adjacent normal tissue sample were collected, and OCT4, SOX2, and NANOG expressions were assessed by immunofluorescent staining. The median follow-up duration was 5.2 years, and the last follow-up date was August 2016.
RESULTS
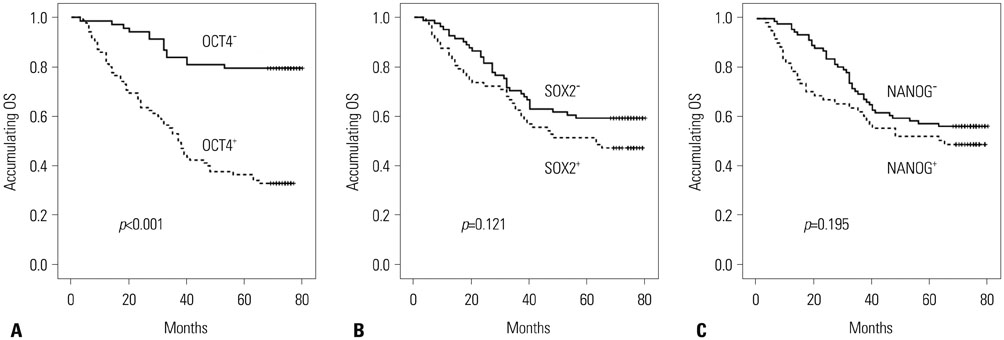
Tumor tissue OCT4 (p < 0.001), SOX2 (p=0.003), and NANOG (p < 0.001) expressions were higher than those in adjacent tissue. OCT4 expression was positively correlated with pathological grade (R=0.185, p=0.022), tumor size (R=0.224, p=0.005), and N stage (R=0.170, p=0.036). NANOG expression was positively associated with tumor size (R=0.169, p=0.036). Kaplan-Meier suggested that OCT4+ was associated with worse OS compared with OCT4− (p < 0.001), while no association of SOX2 (p=0.121) and NANOG expressions (p=0.195) with OS was uncovered. Compared with one or no positive marker, at least two positive markers were associated with shorter OS (p < 0.001), while all three positive markers were correlated with worse OS compared with two or less positive markers (p < 0.001). Multivariate Cox's analysis revealed that OCT4+ (p < 0.001) and N stage (p=0.046) were independent factors for shorter OS.
CONCLUSION
Tumor tissue OCT4 expression was correlated with poor differentiation, tumor size, and N stage, and it can serve as an independent prognostic biomarker in operative patients with RC receiving adjuvant therapy.
Keyword
MeSH Terms
-
Aged
Biomarkers, Tumor/*metabolism
Female
Humans
Male
Multivariate Analysis
Nanog Homeobox Protein/*metabolism
Neoplastic Stem Cells/*metabolism
Octamer Transcription Factor-3/*metabolism
Prognosis
Rectal Neoplasms/*metabolism/pathology/*surgery
SOXB1 Transcription Factors/*metabolism
Survival Analysis
Biomarkers, Tumor
Nanog Homeobox Protein
Octamer Transcription Factor-3
SOXB1 Transcription Factors
Figure
Reference
-
1. Li X, Lu H, Xu K, Wang H, Liang X, Hu Z. Negative lymph node count is an independent prognostic factor for patients with rectal cancer who received preoperative radiotherapy. BMC Cancer. 2017; 17:227.
Article2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30.
Article3. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.
Article4. Hoecht S, Hinkelbein W. Treatment of rectal cancer. N Engl J Med. 2006; 355:2486. author reply 2487-8.
Article5. Beck B, Blanpain C. Unravelling cancer stem cell potential. Nat Rev Cancer. 2013; 13:727–738.
Article6. Cao L, Li C, Shen S, Yan Y, Ji W, Wang J, et al. OCT4 increases BIRC5 and CCND1 expression and promotes cancer progression in hepatocellular carcinoma. BMC Cancer. 2013; 13:82.
Article7. Zhou X, Huang GR, Hu P. Over-expression of Oct4 in human esophageal squamous cell carcinoma. Mol Cells. 2011; 32:39–45.
Article8. Lin J, Zhang L, Huang H, Huang Y, Huang L, Wang J, et al. MiR-26b/KPNA2 axis inhibits epithelial ovarian carcinoma proliferation and metastasis through downregulating OCT4. Oncotarget. 2015; 6:23793–23806.
Article9. Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008; 132:567–582.
Article10. Neumann J, Bahr F, Horst D, Kriegl L, Engel J, Luque RM, et al. SOX2 expression correlates with lymph-node metastases and distant spread in right-sided colon cancer. BMC Cancer. 2011; 11:518.
Article11. Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, et al. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994; 79:643–649.
Article12. Kashyap V, Rezende NC, Scotland KB, Shaffer SM, Persson JL, Gudas LJ, et al. Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs. Stem Cells Dev. 2009; 18:1093–1108.
Article13. Wang YD, Cai N, Wu XL, Cao HZ, Xie LL, Zheng PS. OCT4 promotes tumorigenesis and inhibits apoptosis of cervical cancer cells by miR-125b/BAK1 pathway. Cell Death Dis. 2013; 4:e760.
Article14. Tang YA, Chen CH, Sun HS, Cheng CP, Tseng VS, Hsu HS, et al. Global Oct4 target gene analysis reveals novel downstream PTEN and TNC genes required for drug-resistance and metastasis in lung cancer. Nucleic Acids Res. 2015; 43:1593–1608.
Article15. Wu Y, Liu S, Xin H, Jiang J, Younglai E, Sun S, et al. Up-regulation of microRNA-145 promotes differentiation by repressing OCT4 in human endometrial adenocarcinoma cells. Cancer. 2011; 117:3989–3998.
Article16. Yu B, Cai H, Xu Z, Xu T, Zou Q, Gu M. Expressions of stem cell transcription factors Nanog and Oct4 in renal cell carcinoma tissues and clinical significance. Artif Cells Nanomed Biotechnol. 2016; 44:1818–1823.
Article17. Li X, Wang J, Xu Z, Ahmad A, Li E, Wang Y, et al. Expression of Sox2 and Oct4 and their clinical significance in human non-small-cell lung cancer. Int J Mol Sci. 2012; 13:7663–7675.
Article18. Li C, Yan Y, Ji W, Bao L, Qian H, Chen L, et al. OCT4 positively regulates Survivin expression to promote cancer cell proliferation and leads to poor prognosis in esophageal squamous cell carcinoma. PLoS One. 2012; 7:e49693.
Article19. Matsuoka J, Yashiro M, Sakurai K, Kubo N, Tanaka H, Muguruma K, et al. Role of the stemness factors sox2, oct3/4, and nanog in gastric carcinoma. J Surg Res. 2012; 174:130–135.
Article20. Atlasi Y, Mowla SJ, Ziaee SA, Bahrami AR. OCT-4, an embryonic stem cell marker, is highly expressed in bladder cancer. Int J Cancer. 2007; 120:1598–1602.
Article21. Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, et al. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res. 2008; 14:4085–4095.
Article22. Shen L, Huang X, Xie X, Su J, Yuan J, Chen X. High expression of SOX2 and OCT4 indicates radiation resistance and an independent negative prognosis in cervical squamous cell carcinoma. J Histochem Cytochem. 2014; 62:499–509.
Article23. Linn DE, Yang X, Sun F, Xie Y, Chen H, Jiang R, et al. A role for OCT4 in tumor initiation of drug-resistant prostate cancer cells. Genes Cancer. 2010; 1:908–916.
Article24. Li B, Yao Z, Wan Y, Lin D. Overexpression of OCT4 is associated with gefitinib resistance in non-small cell lung cancer. Oncotarget. 2016; 7:77342–77347.
Article25. Sullivan JP, Minna JD, Shay JW. Evidence for self-renewing lung cancer stem cells and their implications in tumor initiation, progression, and targeted therapy. Cancer Metastasis Rev. 2010; 29:61–72.
Article26. Li W, Zhou Y, Zhang X, Yang Y, Dan S, Su T, et al. Dual inhibiting OCT4 and AKT potently suppresses the propagation of human cancer cells. Sci Rep. 2017; 7:46246.
Article27. Emhemmed F, Ali Azouaou S, Thuaud F, Schini-Kerth V, Désaubry L, Muller CD, et al. Selective anticancer effects of a synthetic flavagline on human Oct4-expressing cancer stem-like cells via a p38 MAPK-dependent caspase-3-dependent pathway. Biochem Pharmacol. 2014; 89:185–196.
Article28. Yong X, Tang B, Xiao YF, Xie R, Qin Y, Luo G, et al. Helicobacter pylori upregulates Nanog and Oct4 via Wnt/β-catenin signaling pathway to promote cancer stem cell-like properties in human gastric cancer. Cancer Lett. 2016; 374:292–303.
Article29. Teng HF, Li PN, Hou DR, Liu SW, Lin CT, Loo MR, et al. Valproic acid enhances Oct4 promoter activity through PI3K/Akt/mTOR pathway activated nuclear receptors. Mol Cell Endocrinol. 2014; 383:147–158.
Article30. Rectal Cancer Treatment (PDQ(R)): Health Professional Version. PDQ Cancer Information Summaries. Bethesda (MD): National Cancer Institute;2002.31. Al-Marzoqee FY, Khoder G, Al-Awadhi H, John R, Beg A, Vincze A, et al. Upregulation and inhibition of the nuclear translocation of Oct4 during multistep gastric carcinogenesis. Int J Oncol. 2012; 41:1733–1743.
Article32. Wen Y, Hou Y, Huang Z, Cai J, Wang Z. SOX2 is required to maintain cancer stem cells in ovarian cancer. Cancer Sci. 2017; 108:719–731.
Article33. Xiao L, Song Y, Huang W, Yang S, Fu J, Feng X, et al. Expression of SOX2, NANOG and OCT4 in a mouse model of lipopolysaccharide-induced acute uterine injury and intrauterine adhesions. Reprod Biol Endocrinol. 2017; 15:14.
Article34. Piva M, Domenici G, Iriondo O, Rábano M, Simöes BM, Comaills V, et al. Sox2 promotes tamoxifen resistance in breast cancer cells. EMBO Mol Med. 2014; 6:66–79.
Article35. Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009; 41:1238–1242.
Article36. Sodja E, Rijavec M, Koren A, Sadikov A, KoroŠec P, Cufer T. The prognostic value of whole blood SOX2, NANOG and OCT4 mRNA expression in advanced small-cell lung cancer. Radiol Oncol. 2016; 50:188–196.
Article37. Yamawaki K, Ishiguro T, Mori Y, Yoshihara K, Suda K, Tamura R, et al. Sox2-dependent inhibition of p21 is associated with poor prognosis of endometrial cancer. Cancer Sci. 2017; 108:632–640.
Article38. Velcheti V, Schalper K, Yao X, Cheng H, Kocoglu M, Dhodapkar K, et al. High SOX2 levels predict better outcome in non-small cell lung carcinomas. PLoS One. 2013; 8:e61427.
Article39. Sholl LM, Barletta JA, Yeap BY, Chirieac LR, Hornick JL. Sox2 protein expression is an independent poor prognostic indicator in stage I lung adenocarcinoma. Am J Surg Pathol. 2010; 34:1193–1198.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The expressions of stem cell markers: Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Tcl1, Tbx3, Dppa4, and Esrrb in bladder, colon, and prostate cancer, and certain cancer cell lines
- Metastasis prognostic factors and cancer stem cell-related transcription factors associated with metastasis induction in canine metastatic mammary gland tumors
- The Role and Specific Mechanism of OCT4 in Cancer Stem Cells: A Review
- OCT4 Expression Enhances Features of Cancer Stem Cells in a Mouse Model of Breast Cancer
- Enhanced proliferation and differentiation of Oct4- and Sox2-overexpressing human adipose tissue mesenchymal stem cells