Allergy Asthma Immunol Res.
2014 Jan;6(1):83-88. 10.4168/aair.2014.6.1.83.
A Novel Synthetic Mycolic Acid Inhibits Bronchial Hyperresponsiveness and Allergic Inflammation in a Mouse Model of Asthma
- Affiliations
-
- 1Asan Institute for Life Sciences, University of Ulsan College of Medicine, Seoul, Korea.
- 2Applied Research Division Neopharm Co., Ltd., Daejeon, Korea.
- 3Department of Pediatrics, Childhood Asthma Atopy Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sjhong@amc.seoul.kr leeyc@chonbuk.ac.kr
- 4Research Center for Standardization of Allergic Diseases, University of Ulsan College of Medicine, Seoul, Korea.
- 5Department of Pediatrics, Korea Cancer Center Hospital, Seoul, Korea.
- 6Department of Pediatrics, Inje University Haeundae Paik Hospital, Busan, Korea.
- 7Research Center for Pulmonary Disorders, Chonbuk National University Medical School, Jeonju, Korea. sjhong@amc.seoul.kr leeyc@chonbuk.ac.kr
- KMID: 2166946
- DOI: http://doi.org/10.4168/aair.2014.6.1.83
Abstract
- PURPOSE
Recognition of microbes is important to trigger the innate immune system. Mycolic acid (MA) is a component of the cell walls of mycobacteria such as Mycobacterium bovis Bacillus Calmette-Guerin. MA has immunogenic properties, which may modulate the innate and adaptive immune response. This study aimed to investigate whether a novel synthetic MA (sMA) inhibits allergic inflammatory responses in a mouse model of asthma.
METHODS
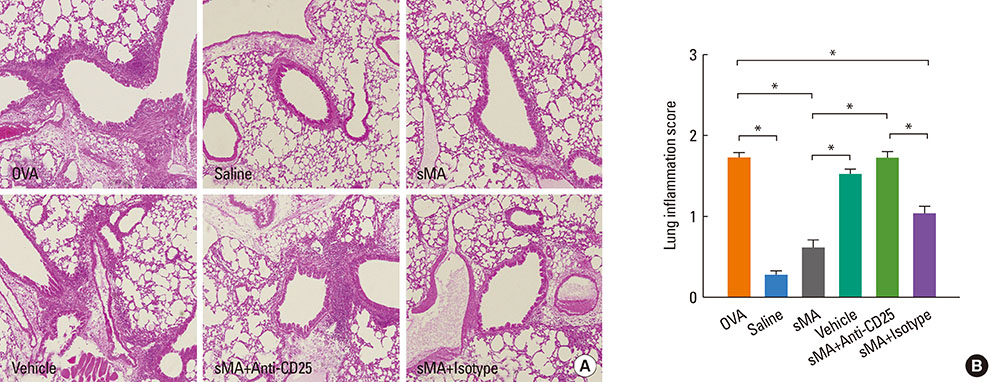
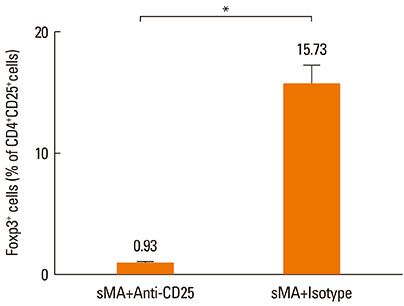
BALB/c mice were injected intraperitoneally with sMA followed by sensitization and challenge with ovalbumin (OVA). Mice were examined for bronchial hyperresponsiveness (BHR), the influx of inflammatory cells into the lung tissues, histopathological changes in the lungs and CD4+CD25+Foxp3+ T cells in the spleen, and examined the response after the depleting regulatory T cells (Tregs) with an anti-CD25mAb.
RESULTS
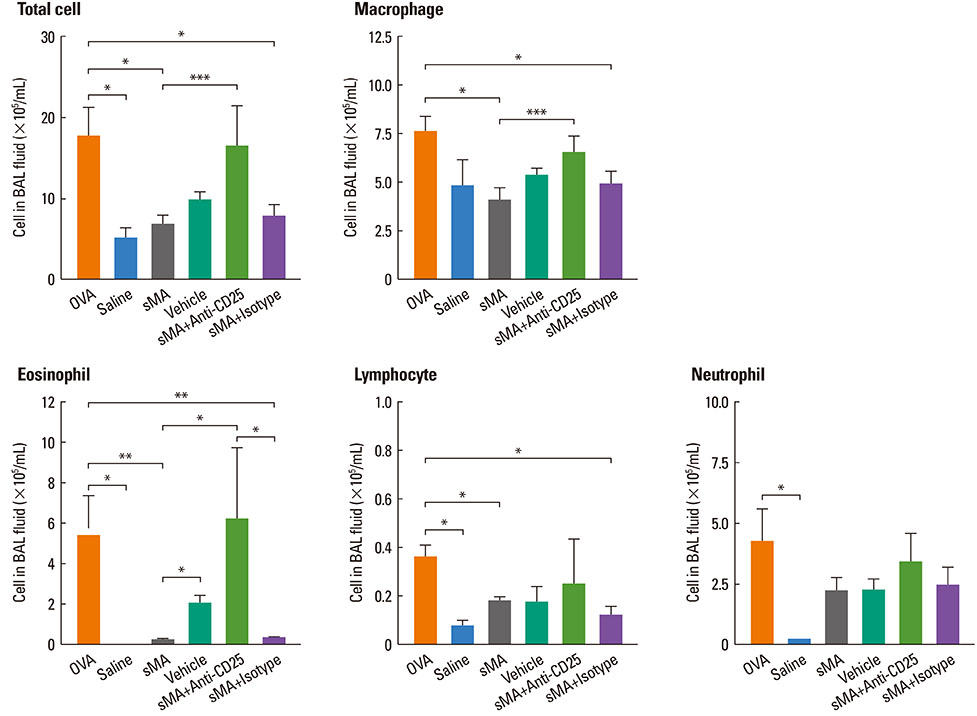
Treatment of mice with sMA suppressed the asthmatic response, including BHR, bronchoalveolar inflammation, and pulmonary eosinophilic inflammation. Anti-CD25mAb treatment abrogated the suppressive effects of sMA in this mouse model of asthma and totally depleted CD4+CD25+Foxp3+ T cells in the spleen.
CONCLUSIONS
sMA attenuated allergic inflammation in a mouse model of asthma, which might be related with CD4+CD25+Foxp3+ T cell.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Tolerogenic Dendritic Cells Reduce Airway Inflammation in a Model of Dust Mite Triggered Allergic Inflammation
Luciana S. Aragão-França, Viviane C. J. Rocha, Andre Cronemberger-Andrade, F. H. B. Costa, José Fernandes Vasconcelos, Daniel Abensur Athanazio, Daniela Nascimento Silva, E. S. Santos, Cássio Santana Meira, C. F. Araújo, Jéssica Vieira Cerqueira, Fabíola Cardillo, Neuza Maria Alcântara-Neves, Milena Botelho Pereira Soares, Lain C. Pontes-de-Carvalho
Allergy Asthma Immunol Res. 2018;10(4):406-419. doi: 10.4168/aair.2018.10.4.406.
Reference
-
1. Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow JC Jr. Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992; 146:109–115.2. Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006; 355:2226–2235.3. Hong SJ, Ahn KM, Lee SY, Kim KE. The prevalences of asthma and allergic diseases in Korean children. Korean J Pediatr. 2008; 51:343–350.4. Lee SI. Prevalence of childhood asthma in Korea: international study of asthma and allergies in childhood. Allergy Asthma Immunol Res. 2010; 2:61–64.5. Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989; 299:1259–1260.6. Riedler J, Braun-Fahrländer C, Eder W, Schreuer M, Waser M, Maisch S, Carr D, Schierl R, Nowak D, von Mutius E. ALEX Study Team. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001; 358:1129–1133.7. Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrländer C, Heederik D, Piarroux R, von Mutius E. GABRIELA Transregio 22 Study Group. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011; 364:701–709.8. Korf J, Stoltz A, Verschoor J, De Baetselier P, Grooten J. The Mycobacterium tuberculosis cell wall component mycolic acid elicits pathogen-associated host innate immune responses. Eur J Immunol. 2005; 35:890–900.9. Obihara CC, Beyers N, Gie RP, Potter PC, Marais BJ, Lombard CJ, Enarson DA, Kimpen JL. Inverse association between Mycobacterium tuberculosis infection and atopic rhinitis in children. Allergy. 2005; 60:1121–1125.10. Korf JE, Pynaert G, Tournoy K, Boonefaes T, Van Oosterhout A, Ginneberge D, Haegeman A, Verschoor JA, De Baetselier P, Grooten J. Macrophage reprogramming by mycolic acid promotes a tolerogenic response in experimental asthma. Am J Respir Crit Care Med. 2006; 174:152–160.11. Lee M, Kim MK, Kwon MJ, Park BD, Kim MH, Goodfellow M, Lee ST. Effect of the synthesized mycolic acid on the biodegradation of diesel oil by Gordonia nitida strain LE31. J Biosci Bioeng. 2005; 100:429–436.12. Yu J, Jang SO, Kim BJ, Song YH, Kwon JW, Kang MJ, Choi WA, Jung HD, Hong SJ. The effects of Lactobacillus rhamnosus on the prevention of asthma in a murine model. Allergy Asthma Immunol Res. 2010; 2:199–205.13. Tournoy KG, Kips JC, Schou C, Pauwels RA. Airway eosinophilia is not a requirement for allergen-induced airway hyperresponsiveness. Clin Exp Allergy. 2000; 30:79–85.14. Sayers I, Severn W, Scanga CB, Hudson J, Le Gros G, Harper JL. Suppression of allergic airway disease using mycobacterial lipoglycans. J Allergy Clin Immunol. 2004; 114:302–309.15. Ito T, Hasegawa A, Hosokawa H, Yamashita M, Motohashi S, Naka T, Okamoto Y, Fujita Y, Ishii Y, Taniguchi M, Yano I, Nakayama T. Human Th1 differentiation induced by lipoarabinomannan/lipomannan from Mycobacterium bovis BCG Tokyo-172. Int Immunol. 2008; 20:849–860.16. Bang BR, Chun E, Shim EJ, Lee HS, Lee SY, Cho SH, Min KU, Kim YY, Park HW. Alveolar macrophages modulate allergic inflammation in a murine model of asthma. Exp Mol Med. 2011; 43:275–280.17. Tsuji S, Matsumoto M, Takeuchi O, Akira S, Azuma I, Hayashi A, Toyoshima K, Seya T. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guérin: involvement of toll-like receptors. Infect Immun. 2000; 68:6883–6890.18. Fehérvari Z, Sakaguchi S. Development and function of CD25+CD4+ regulatory T cells. Curr Opin Immunol. 2004; 16:203–208.19. Vander Beken S, Al Dulayymi JR, Naessens T, Koza G, Maza-Iglesias M, Rowles R, Theunissen C, De Medts J, Lanckacker E, Baird MS, Grooten J. Molecular structure of the Mycobacterium tuberculosis virulence factor, mycolic acid, determines the elicited inflammatory pattern. Eur J Immunol. 2011; 41:450–460.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship Between Atopy and Bronchial Hyperresponsiveness
- The effect of immunotherapy on nonspecific bronchial hyperresponsiveness in bronchial asthma and allergic rhinitis
- The Effectiveness of Pimecrolimus in Airway Inflammation and Bronchial Hyperresponsiveness in Murine Asthma Model
- Understanding the Mouse Model of Respiratory Allergic Diseases
- Effect of Coexistence of Allergic Rhinitis in Mild Persistent Asthma on Lower Airway Eosinophilic Inflammation