Allergy Asthma Immunol Res.
2014 Jan;6(1):66-74. 10.4168/aair.2014.6.1.66.
Stimulated Bronchial Epithelial Cells Release Bioactive Lysophosphatidylcholine 16:0, 18:0, and 18:1
- Affiliations
-
- 1Department of Pharmacology, Rush University Medical Center, Chicago, IL, USA. hlum@rush.edu
- 2Department of Medicinal Chemistry & Pharmacognosy, University of Illinois, Chicago, IL, USA.
- 3Department of Medicine, Rush University Medical Center, Chicago, IL, USA.
- KMID: 2166940
- DOI: http://doi.org/10.4168/aair.2014.6.1.66
Abstract
- PURPOSE
In human subjects and animal models with acute and chronic lung injury, the bioactive lysophosphatidylcholine (LPC) is elevated in lung lining fluids. The increased LPC can promote an inflammatory microenvironment resulting in lung injury. Furthermore, pathological lung conditions are associated with upregulated phospholipase A2 (PLA2), the predominant enzyme producing LPC in tissues by hydrolysis of phosphatidylcholine. However, the lung cell populations responsible for increases of LPC have yet to be systematically characterized. The goal was to investigate the LPC generation by bronchial epithelial cells in response to pathological mediators and determine the major LPC species produced.
METHODS
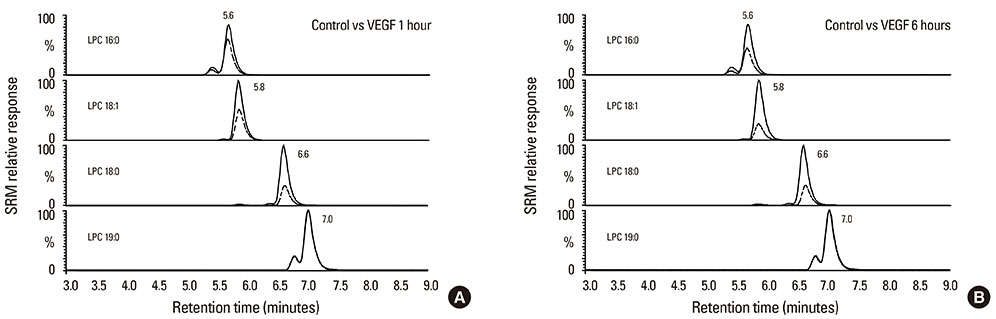
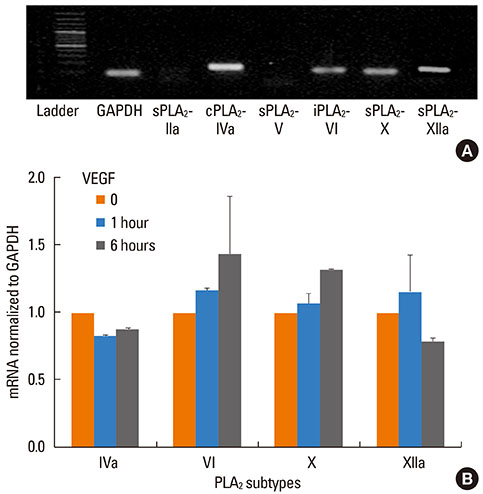
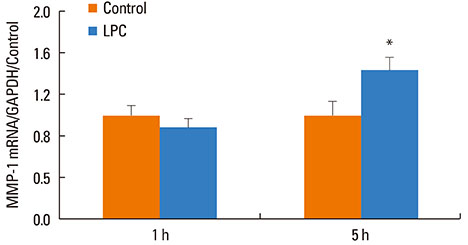
Primary human bronchial epithelial cells (NHBE) were challenged by vascular endothelial growth factor (VEGF) for 1 or 6 h, and condition medium and cells collected for quantification of predominant LPC species by high performance liquid chromatography-tandem mass spectrometry (LC-MS-MS). The cells were analyzed by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) for PLA2. The direct effects of LPC in inducing inflammatory activities on NHBE were assessed by transepithelial resistance as well as expression of interleukin-8 (IL-8) and matrix metalloproteinase-1 (MMP-1).
RESULTS
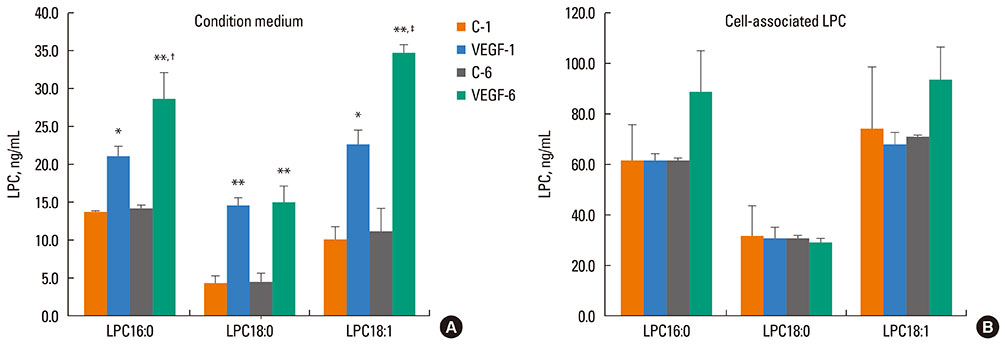
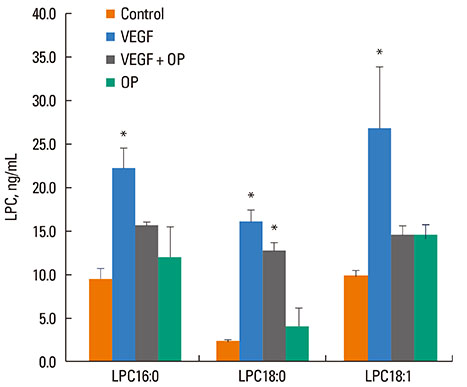
VEGF stimulation of NHBE for 1 or 6 h, significantly increased concentrations of LPC16:0, LPC18:0, and LPC18:1 in condition medium compared to control. The sPLA2-selective inhibitor (oleyloxyethyl phosphorylcholine) inhibited the VEGF-induced release of LPC16:0 and LPC18:1 and PLA2 activity. In contrast, NHBE stimulated with TNF did not induce LPC release. VEGF did not increase mRNA of PLA2 subtypes sPLA2-X, sPLA2-XIIa, cPLA2-IVa, and iPLA2-VI. Exogenous LPC treatment increased expression of IL-8 and MMP-1, and reduced the transepithelial resistance in NHBE.
CONCLUSIONS
Our findings indicate that VEGF-stimulated bronchial epithelial cells are a key source of extracellular LPCs, which can function as an autocrine mediator with potential to induce airway epithelial inflammatory injury.
MeSH Terms
-
Epithelial Cells*
Group X Phospholipases A2
Humans
Hydrolysis
Interleukin-8
Lung
Lung Injury
Lysophosphatidylcholines*
Mass Spectrometry
Matrix Metalloproteinase 1
Models, Animal
Phosphatidylcholines
Phospholipases A2
Reverse Transcriptase Polymerase Chain Reaction
RNA, Messenger
Vascular Endothelial Growth Factor A
Group X Phospholipases A2
Interleukin-8
Lysophosphatidylcholines
Matrix Metalloproteinase 1
Phosphatidylcholines
Phospholipases A2
RNA, Messenger
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Chilton FH, Averill FJ, Hubbard WC, Fonteh AN, Triggiani M, Liu MC. Antigen-induced generation of lyso-phospholipids in human airways. J Exp Med. 1996; 183:2235–2245.2. Nakos G, Kitsiouli EI, Tsangaris I, Lekka ME. Bronchoalveolar lavage fluid characteristics of early intermediate and late phases of ARDS. Alterations in leukocytes, proteins, PAF and surfactant components. Intensive Care Med. 1998; 24:296–303.3. Arbibe L, Koumanov K, Vial D, Rougeot C, Faure G, Havet N, Longacre S, Vargaftig BB, Béréziat G, Voelker DR, Wolf C, Touqui L. Generation of lyso-phospholipids from surfactant in acute lung injury is mediated by type-II phospholipase A2 and inhibited by a direct surfactant protein A-phospholipase A2 protein interaction. J Clin Invest. 1998; 102:1152–1160.4. Rice KL, Duane PG, Archer SL, Gilboe DP, Niewoehner DE. H2O2 injury causes Ca(2+)-dependent and -independent hydrolysis of phosphatidylcholine in alveolar epithelial cells. Am J Physiol. 1992; 263:L430–L438.5. Schaefer CA, Kuhlmann CR, Gast C, Weiterer S, Li F, Most AK, Neumann T, Backenkohler U, Tillmanns H, Waldecker B, Wiecha J, Erdogan A. Statins prevent oxidized low-density lipoprotein- and lysophosphatidylcholine-induced proliferation of human endothelial cells. Vascul Pharmacol. 2004; 41:67–73.6. Radu CG, Yang LV, Riedinger M, Au M, Witte ON. T cell chemotaxis to lysophosphatidylcholine through the G2A receptor. Proc Natl Acad Sci U S A. 2004; 101:245–250.7. Kohno M, Yokokawa K, Yasunari K, Minami M, Kano H, Hanehira T, Yoshikawa J. Induction by lysophosphatidylcholine, a major phospholipid component of atherogenic lipoproteins, of human coronary artery smooth muscle cell migration. Circulation. 1998; 98:353–359.8. Liu-Wu Y, Hurt-Camejo E, Wiklund O. Lysophosphatidylcholine induces the production of IL-1beta by human monocytes. Atherosclerosis. 1998; 137:351–357.9. Spangelo BL, Jarvis WD. Lysophosphatidylcholine stimulates interleukin-6 release from rat anterior pituitary cells in vitro. Endocrinology. 1996; 137:4419–4426.10. Takeshita S, Inoue N, Gao D, Rikitake Y, Kawashima S, Tawa R, Sakurai H, Yokoyama M. Lysophosphatidylcholine enhances superoxide anions production via endothelial NADH/NADPH oxidase. J Atheroscler Thromb. 2000; 7:238–246.11. Zou Y, Kim CH, Chung JH, Kim JY, Chung SW, Kim MK, Im DS, Lee J, Yu BP, Chung HY. Upregulation of endothelial adhesion molecules by lysophosphatidylcholine. Involvement of G protein-coupled receptor GPR4. FEBS J. 2007; 274:2573–2584.12. Nishiyama O, Kume H, Kondo M, Ito Y, Ito M, Yamaki K. Role of lysophosphatidylcholine in eosinophil infiltration and resistance in airways. Clin Exp Pharmacol Physiol. 2004; 31:179–184.13. Niewoehner DE, Rice K, Sinha AA, Wangensteen D. Injurious effects of lysophosphatidylcholine on barrier properties of alveolar epithelium. J Appl Physiol. 1987; 63:1979–1986.14. Lindahl M, Hede AR, Tagesson C. Lysophosphatidylcholine increases airway and capillary permeability in the isolated perfused rat lung. Exp Lung Res. 1986; 11:1–12.15. Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009; 50:Suppl. S237–S242.16. Burke JE, Dennis EA. Phospholipase A2 biochemistry. Cardiovasc Drugs Ther. 2009; 23:49–59.17. Masuda S, Murakami M, Mitsuishi M, Komiyama K, Ishikawa Y, Ishii T, Kudo I. Expression of secretory phospholipase A2 enzymes in lungs of humans with pneumonia and their potential prostaglandin-synthetic function in human lung-derived cells. Biochem J. 2005; 387:27–38.18. Bowton DL, Seeds MC, Fasano MB, Goldsmith B, Bass DA. Phospholipase A2 and arachidonate increase in bronchoalveolar lavage fluid after inhaled antigen challenge in asthmatics. Am J Respir Crit Care Med. 1997; 155:421–425.19. Hallstrand TS, Chi EY, Singer AG, Gelb MH, Henderson WR Jr. Secreted phospholipase A2 group X overexpression in asthma and bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2007; 176:1072–1078.20. Nakano T, Arita H. Enhanced expression of group II phospholipase A2 gene in the tissues of endotoxin shock rats and its suppression by glucocorticoid. FEBS Lett. 1990; 273:23–26.21. Ljungman AG, Tagesson C, Lindahl M. Endotoxin stimulates the expression of group II PLA2 in rat lung in vivo and in isolated perfused lungs. Am J Physiol. 1996; 270:L752–L760.22. Lopez-Guisa JM, Powers C, File D, Cochrane E, Jimenez N, Debley JS. Airway epithelial cells from asthmatic children differentially express proremodeling factors. J Allergy Clin Immunol. 2012; 129:990–997.e6.23. Braber S, Henricks PA, Nijkamp FP, Kraneveld AD, Folkerts G. Inflammatory changes in the airways of mice caused by cigarette smoke exposure are only partially reversed after smoking cessation. Respir Res. 2010; 11:99.24. Vasakova M, Sterclova M, Kolesar L, Slavcev A, Pohunek P, Sulc J, Skibova J, Striz I. Bronchoalveolar lavage fluid cellular characteristics, functional parameters and cytokine and chemokine levels in interstitial lung diseases. Scand J Immunol. 2009; 69:268–274.25. Zhang J, Zheng L, Bai M. Dynamic expression of tumor necrosis factor-alpha and vascular endothelial growth factor in rat model of pulmonary emphysema induced by smoke exposure. J Huazhong Univ Sci Technolog Med Sci. 2007; 27:505–507.26. Qiao J, Huang F, Naikawadi RP, Kim KS, Said T, Lum H. Lysophosphatidylcholine impairs endothelial barrier function through the G protein-coupled receptor GPR4. Am J Physiol Lung Cell Mol Physiol. 2006; 291:L91–101.27. Huang F, Subbaiah PV, Holian O, Zhang J, Johnson A, Gertzberg N, Lum H. Lysophosphatidylcholine increases endothelial permeability: role of PKCalpha and RhoA cross talk. Am J Physiol Lung Cell Mol Physiol. 2005; 289:L176–L185.28. Huang F, Mehta D, Predescu S, Kim KS, Lum H. A novel lysophospholipid-and pH-sensitive receptor, GPR4, in brain endothelial cells regulates monocyte transmigration. Endothelium. 2007; 14:25–34.29. Aiyar N, Disa J, Ao Z, Ju H, Nerurkar S, Willette RN, Macphee CH, Johns DG, Douglas SA. Lysophosphatidylcholine induces inflammatory activation of human coronary artery smooth muscle cells. Mol Cell Biochem. 2007; 295:113–120.30. Han KH, Hong KH, Ko J, Rhee KS, Hong MK, Kim JJ, Kim YH, Park SJ. Lysophosphatidylcholine up-regulates CXCR4 chemokine receptor expression in human CD4 T cells. J Leukoc Biol. 2004; 76:195–202.31. Murugesan G, Sandhya Rani MR, Gerber CE, Mukhopadhyay C, Ransohoff RM, Chisolm GM, Kottke-Marchant K. Lysophosphatidylcholine regulates human microvascular endothelial cell expression of chemokines. J Mol Cell Cardiol. 2003; 35:1375–1384.32. Silliman CC, Elzi DJ, Ambruso DR, Musters RJ, Hamiel C, Harbeck RJ, Paterson AJ, Bjornsen AJ, Wyman TH, Kelher M, England KM, McLaughlin-Malaxecheberria N, Barnett CC, Aiboshi J, Bannerjee A. Lysophosphatidylcholines prime the NADPH oxidase and stimulate multiple neutrophil functions through changes in cytosolic calcium. J Leukoc Biol. 2003; 73:511–524.33. Zhu X, Learoyd J, Butt S, Zhu L, Usatyuk PV, Natarajan V, Munoz NM, Leff AR. Regulation of eosinophil adhesion by lysophosphatidylcholine via a non-store-operated Ca2+ channel. Am J Respir Cell Mol Biol. 2007; 36:585–593.34. Jino H, Kurahashi K, Usui H, Nakata Y, Shimizu Y. Possible involvement of endothelial leukotrienes in acetylcholine-induced contraction in rabbit coronary artery. Life Sci. 1996; 59:961–967.35. Sim SS, Choi JC, Min DS, Rhie DJ, Yoon SH, Hahn SJ, Kim CJ, Kim MS, Jo YH. The involvement of phospholipase A(2) in ethanol-induced gastric muscle contraction. Eur J Pharmacol. 2001; 413:281–285.36. Daray FM, Colombo JR, Kibrik JR, Errasti AE, Pelorosso FG, Nowak W, Cracowski JL, Rothlin RP. Involvement of endothelial thromboxane A2 in the vasoconstrictor response induced by 15-E2t-isoprostane in isolated human umbilical vein. Naunyn Schmiedebergs Arch Pharmacol. 2006; 373:367–375.37. Xing F, Liu J, Mo Y, Liu Z, Qin Q, Wang J, Fan Z, Long Y, Liu N, Zhao K, Jiang Y. Lysophosphatidylcholine up-regulates human endothelial nitric oxide synthase gene transactivity by c-Jun N-terminal kinase signalling pathway. J Cell Mol Med. 2009; 13:1136–1148.38. Masamune A, Sakai Y, Yoshida M, Satoh A, Satoh K, Shimosegawa T. Lysophosphatidylcholine activates transcription factor NF-kappaB and AP-1 in AR42J cells. Dig Dis Sci. 2001; 46:1871–1881.39. Ueno Y, Kume N, Miyamoto S, Morimoto M, Kataoka H, Ochi H, Nishi E, Moriwaki H, Minami M, Hashimoto N, Kita T. Lysophosphatidylcholine phosphorylates CREB and activates the jun2TRE site of c-jun promoter in vascular endothelial cells. FEBS Lett. 1999; 457:241–245.40. Fang X, Gibson S, Flowers M, Furui T, Bast RC Jr, Mills GB. Lysophosphatidylcholine stimulates activator protein 1 and the c-Jun N-terminal kinase activity. J Biol Chem. 1997; 272:13683–13689.41. Butler BD, Davies I, Drake RE. Effect of lysophosphatidylcholine on the filtration coefficient in intact dog lungs. Am J Physiol. 1989; 257:H1466–H1470.42. Simet SM, Wyatt TA, DeVasure J, Yanov D, Allen-Gipson D, Sisson JH. Alcohol increases the permeability of airway epithelial tight junctions in Beas-2B and NHBE cells. Alcohol Clin Exp Res. 2012; 36:432–442.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Comparison of the Effect of Cigarette and Stop Smoking-aiding Cigarette on Release of IL-6 from Bronchial Epithelial Cell
- The effect of rhinovirus and cigarette smoke extract on the production of interleukin-8 in human bronchial epithelial cells
- Effects of Sphingosine-1-phosphate, Furosemide and Indomethacin on Mucin Release from Airway Goblet Cells
- CXC and CC Chemokine Expression by Intestinal Epithelial Cells in Response to Clostridium difficile Toxin A
- IL-1Ra Elaboration by Colchicine Stimulation in Normal Human Bronchial Epithelial Cells