Cancer Res Treat.
2006 Jun;38(3):152-158.
Inhibition of Phorbol Ester-induced Mouse Skin Tumor Promotion and COX-2 Expression by Celecoxib: C/EBP as a Potential Molecular Target
- Affiliations
-
- 1National Research Laboratory of Molecular Carcinogenesis and Chemoprevention, College of Pharmacy, Seoul National University, Korea. surh@plaza.snu.ac.kr
- 2College of Dentistry, Yonsei University, Seoul, Korea.
Abstract
-
PURPOSE: Inflammation acts as a driving force for the development of cancer. Multiple lines of evidence suggest that nonsteroidal anti-inflammatory drugs, especially those that specifically target cyclooxygenase-2 (COX-2), are effective in preventing certain cancers. The present study was aimed at investigating the antitumor promoting potential of celecoxib in chemically induced mouse skin tumorigenesis, as well as elucidating the underlying molecular mechanisms.
MATERIALS AND METHODS
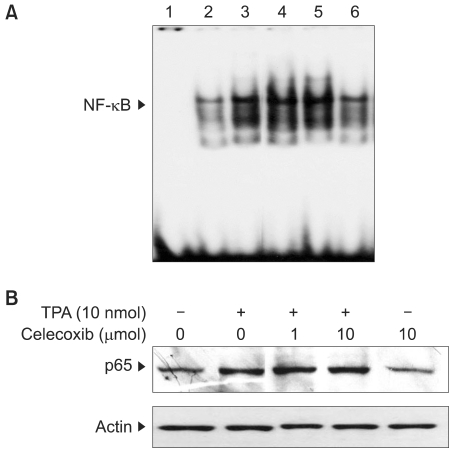
To study the antitumor promoting effects of celecoxib, we used the classical two-stage mouse skin tumorigenesis model that involves initiation with a single application of 7,12-dimethylbenz[alpha]anthracene (DMBA) followed by promotion with repeated applications of 12-O-tetradecanoylphorbol-13-acetate (TPA). The effects of celecoxib on the expression of COX-2, vascular endothelial growth factor (VEGF), p65 and the different isoforms of CCAAT/enhancer binding protein (C/EBP) were examined by performing Western blot analysis. Electrophoretic mobility gel shift assay was used to examine the effects of celecoxib on the TPA-induced DNA binding activities of various transcription factors.
RESULTS
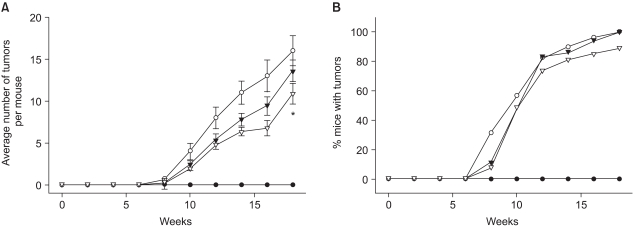
Our study revealed that topical application of celecoxib (10 micromol) significantly reduced the multiplicity of papillomas in DMBA-initiated and TPA-promoted mouse skin. Pretreatment with celecoxib also diminished the expression of COX-2 and VEGF in the mouse skin papillomas. Pretreatment with celecoxib attenuated DNA binding of transcription factor (C/EBP) in the TPA-stimulated mouse skin. Moreover, celecoxib suppressed the TPA-induced nuclear expression of C/EBPdelta, but not C/EBPbeta, in mouse skin in vivo.
CONCLUSION
Our study demonstrates the inhibitory effects of celecoxib on mouse skin tumor promotion, which was associated with a decreased expression of COX-2 and VEGF, as well as inhibition of C/EBP activation.
Keyword
MeSH Terms
-
Animals
Blotting, Western
Carcinogenesis
Carrier Proteins
Chemoprevention
Cyclooxygenase 2
DNA
Inflammation
Mice*
NF-kappa B
Papilloma
Protein Isoforms
Skin*
Transcription Factors
Vascular Endothelial Growth Factor A
Celecoxib
Carrier Proteins
Cyclooxygenase 2
DNA
NF-kappa B
Protein Isoforms
Transcription Factors
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin. 2006; 56:69–83. PMID: 16514135.
Article2. Furstenberger G, Gross M, Marks F. Eicosanoids and multistage carcinogenesis in NMRI mouse skin: role of prostaglandins E and F in conversion (first stage of tumor promotion) and promotion (second stage of tumor promotion). Carcinogenesis. 1989; 10:91–96. PMID: 2910536.
Article3. Muller-Decker K, Neufang G, Berger I, Neumann M, Marks F, Furstenberger G. Transgenic cyclooxygenase-2 overexpression sensitizes mouse skin for carcinogenesis. Proc Natl Acad Sci USA. 2002; 99:12483–12488. PMID: 12221288.
Article4. Tiano HF, Loftin CD, Akunda J, Lee CA, Spalding J, Sessoms A, et al. Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer Res. 2002; 62:3395–3401. PMID: 12067981.5. Millan O, Rico D, Peinado H, Zarich N, Stamatakis K, Perez-Sala D, et al. Potentiation of tumor formation by topical administration of 15-deoxy-delta12,14-prostaglandin J2 in a model of skin carcinogenesis. Carcinogenesis. 2006; 27:328–336. PMID: 16113051.6. Chun KS, Keum YS, Han SS, Song YS, Kim SH, Surh YJ. Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extracellular signal-regulated kinase activity and NF-κB activation. Carcinogenesis. 2003; 24:1515–1524. PMID: 12844482.
Article7. Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000; 342:1946–1952. PMID: 10874062.
Article8. Chun KS, Surh YJ. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem Pharmacol. 2004; 68:1089–1100. PMID: 15313405.
Article9. Chun KS, Kim SH, Song YS, Surh YJ. Celecoxib inhibits phorbol ester-induced expression of COX-2 and activation of AP-1 and p38 MAP kinase in mouse skin. Carcinogenesis. 2004; 25:713–722. PMID: 14729583.
Article10. Fujimoto J, Toyoki H, Sakaguchi H, Jahan I, Alam SM, Tamaya T. Clinical implications of expression of cyclooxygenase-2 related to angiogenesis in ovarian cancer. Oncol Rep. 2006; 15:21–25. PMID: 16328030.
Article11. Wu G, Luo J, Rana JS, Laham R, Sellke FW, Li J. Involvement of COX-2 in VEGF-induced angiogenesis via P38 and JNK pathways in vascular endothelial cells. Cardiovasc Res. 2006; 69:512–519. PMID: 16336951.
Article12. Cardones AR, Banez LL. VEGF inhibitors in cancer therapy. Curr Pharm Des. 2006; 12:387–394. PMID: 16454752.
Article13. Kim Y, Fischer SM. Transcriptional regulation of cyclooxygenase-2 in mouse skin carcinoma cells. Regulatory role of CCAAT/enhancer-binding proteins in the differential expression of cyclooxygenase-2 in normal and neoplastic tissues. J Biol Chem. 1998; 273:27686–27694. PMID: 9765305.14. Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nature Rev Cancer. 2006; 6:130–140. PMID: 16491072.
Article15. Solomon DH, Avorn J, Sturmer T, Glynn RJ, Mogun H, Schneeweiss S. Cardiovascular outcomes in new users of coxibs and nonsteroidal antiinflammatory drugs: high-risk subgroups and time course of risk. Arthritis Rheum. 2006; 54:1378–1389. PMID: 16645966.
Article16. Motsko SP, Rascati KL, Busti AJ, Wilson JP, Barner JC, Lawson KA, et al. Temporal relationship between use of NSAIDs, including selective COX-2 inhibitors, and cardiovascular risk. Drug Saf. 2006; 29:621–632. PMID: 16808554.
Article17. Sooriakumaran P. COX-2 inhibitors and the heart: are all coxibs the same? Postgrad Med J. 2006; 82:242–245. PMID: 16597810.
Article18. Hawkey CJ, Fortun PJ. Cyclooxygenase-2 inhibitors. Curr Opin Gastroenterol. 2005; 21:660–664. PMID: 16220041.
Article19. Andersohn F, Schade R, Suissa S, Garbe E. Cyclooxygenase-2 selective nonsteroidal anti-inflammatory drugs and the risk of ischemic stroke: a nested case-control study. Stroke. 2006; 37:1725–1730. PMID: 16728684.20. Surh YJ, Kundu JK. Signal transduction network leading to COX-2 induction: a road map in search of cancer chemopreventives. Arch Pharm Res. 2005; 28:1–15. PMID: 15742801.
Article21. Grosch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst. 2006; 98:736–747. PMID: 16757698.22. Wei D, Wang L, He Y, Xiong HQ, Abbruzzese JL, Xie K. Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer Res. 2004; 64:2030–2038. PMID: 15026340.
Article23. Amrite AC, Ayalasomayajula SP, Cheruvu NP, Kompella UB. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest Ophthalmol Vis Sci. 2006; 47:1149–1160. PMID: 16505053.24. Inoue H, Yokoyama C, Hara S, Tone Y, Tanabe T. Transcriptional regulation of human prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharide and phorbol ester in vascular endothelial cells. Involvement of both nuclear factor for interleukin-6 expression site and cAMP response element. J Biol Chem. 1995; 270:24965–24971. PMID: 7559624.25. Uto T, Fujii M, Hou DX. Inhibition of lipopolysaccharide-induced cyclooxygenase-2 transcription by 6-(methylsulfinyl) hexyl isothiocyanate, a chemopreventive compound from Wasabia japonica (Miq.) Matsumura, in mouse macrophages. Biochem Pharmacol. 2005; 70:1772–1784. PMID: 16256955.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Growth inhibition of oral squamous cell carcinorma cell line induced by cox inhibitor
- Celecoxib induces cell death on non-small cell lung cancer cells through endoplasmic reticulum stress
- COX-2 inhibitor induced apoptosis in oral squamous cell carcinoma cell line through AKT pathway
- Change of the Invasiveness with Selective Cox-2 Inhibition in an Oral Squamous Cell Carcinoma Cell Line, KB; Preliminary in Vitro Study
- Effects of Genetic and Pharmacologic Inhibition of COX-2 on Colitis-associated Carcinogenesis in Mice