Cancer Res Treat.
2007 Dec;39(4):175-180.
Optimization and Limitation of Calcium Ionophore to Generate DCs from Acute Myeloid Leukemic Cells
- Affiliations
-
- 1Clinical Vaccine R&D Center, Chonnam National University, Hwasun, Jeollanado, Korea.
- 2Department of Hematology-Oncology, Chonnam National University Hwasun Hospital, Hwasun, Jeollanamdo, Korea. drjejung@chonnam.ac.kr
Abstract
-
PURPOSE: Calcium ionophore (CI) is used to generate dendritic cells (DCs) from progenitor cells, monocytes, or leukemic cells. The aim of this study was to determine the optimal dose of CI and the appropriate length of cell culture required for acute myeloid leukemia (AML) cells and to evaluate the limitations associated with CI.
MATERIALS AND METHODS
To generate leukemic DCs, leukemic cells (4 x 10(6) cells) from six AML patients were cultured with various concentrations of CI and/or IL-4 for 1, 2 or 3 days.
RESULTS
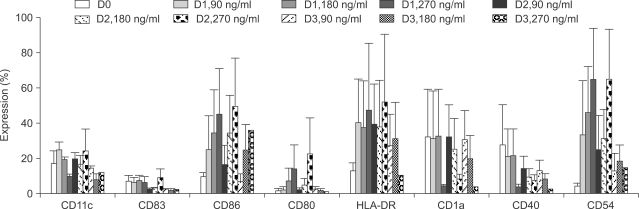
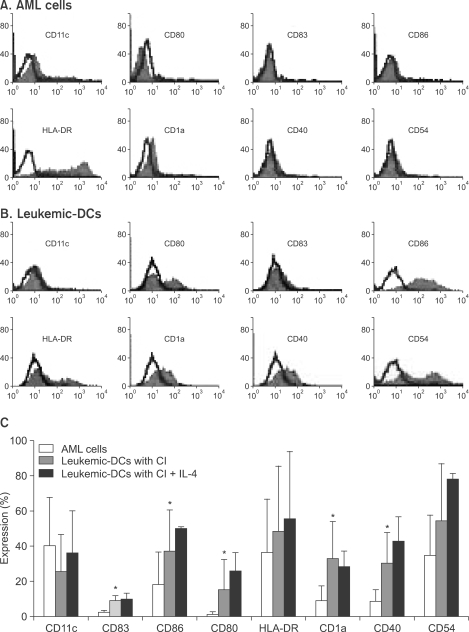
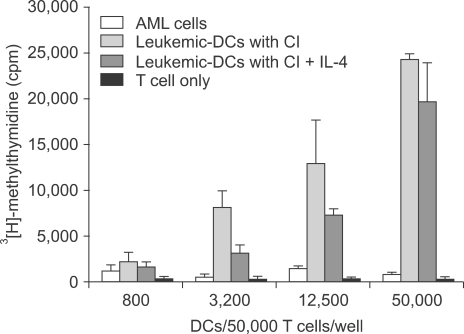
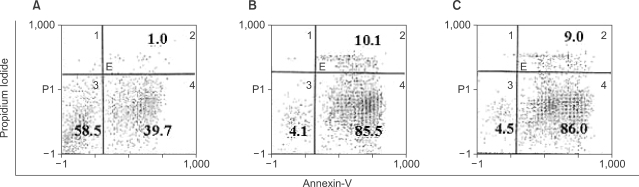
Potent leukemic DCs were successfully generated from all AML patients, with an average number of 1.2 x 10(6) cells produced in the presence of CI (270 ng/ml) for 2 days. Several surface molecules were clearly upregulated in AML cells supplemented with CI and IL-4, but not CD11c. Leukemic DCs cultured with CI had a higher allogeneic T cell stimulatory capacity than untreated AML cells, but the addition of IL-4 did not augment the MLR activity of these cells. AML cells cultured with CI in the presence or absence of IL-4 showed increased levels of apoptosis in comparison to primary cultures of AML cells.
CONCLUSION
Although CI appears to be advantageous in terms of time and cost effectiveness, the results of the present study suggest that the marked induction of apoptosis by CI limits its application to the generation of DCs from AML cells.
MeSH Terms
Figure
Reference
-
1. Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999; 341:1051–1062. PMID: 10502596.
Article2. Buggins AG, Lea N, Gaken J, Darling D, Farzaneh F, Mufti GJ, et al. Effect of costimulation and the microenvironment on antigen presentation by leukemic cells. Blood. 1999; 94:3479–3490. PMID: 10552958.
Article3. Notter M, Willinger T, Erben U, Thiel E. Targeting of a B7-1 (CD80) immunoglobulin G fusion protein to acute myeloid leukemia blasts increases their costimulatory activity for autologous remission T cells. Blood. 2001; 97:3138–3145. PMID: 11342441.
Article4. Santiago-Schwarz F, Coppock DL, Hindenburg AA, Kern J. Identification of a malignant counterpart of the monocytedendritic cell progenitor in an acute myeloid leukemia. Blood. 1994; 84:3054–3062. PMID: 7949177.
Article5. Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997; 90:3245–3287. PMID: 9345009.
Article6. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998; 392:245–252. PMID: 9521319.
Article7. Harrison BD, Adams JA, Briggs M, Brereton ML, Yin JA. Stimulation of autologous proliferative and cytotoxic T-cell responses by leukemic dendritic cells derived from blast cells in acute myeloid leukemia. Blood. 2001; 97:2764–2771. PMID: 11313269.
Article8. Lee JJ, Choi BH, Nam JH, Park MS, Song WH, Yang DH, et al. The generation of leukemic dendritic cells from acute myeloid leukemia cells is potentiated by the addition of CD40L at the terminal maturation stage. J Clin Apher. 2004; 19:130–136. PMID: 15493054.
Article9. Reid DC. Dendritic cells and immunotherapy for malignant disease. Br J Haematol. 2001; 112:874–887. PMID: 11298582.
Article10. Waclavicek M, Berer A, Oehler L, Stockl J, Schloegl E, Majdic O, et al. Calcium ionophore: a single reagent for the differentiation of primary human acute myelogenous leukaemia cells towards dendritic cells. Br J Haematol. 2001; 114:466–473. PMID: 11529871.
Article11. Galea-Lauri J. Immunological weapons against acute myeloid leukemia. Immunology. 2002; 107:20–27. PMID: 12225359.12. Lee JJ, Park MS, Park JS, Kang HK, Kim SK, Nguyen Pham TN, et al. Induction of leukemic-cell-specific cytotoxic T lymphocytes by autologous monocyte-derived dendritic cells presenting leukemic cell antigens. J Clin Apher. 2006; 21:188–194. PMID: 16570260.
Article13. Czerniecki BJ, Carter C, Rivoltini L, Koski GK, Kim HI, Weng DE, et al. Calcium ionophore-treated peripheral blood monocytes and dendritic cells rapidly display characteristics of activated dendritic cells. J Immunol. 1997; 159:3823–3837. PMID: 9378970.14. Engels FH, Koski GK, Bedrosian I, Xu S, Luger S, Nowell PC, et al. Calcium signaling induces acquisition of dendritic cell characteristics in chronic myelogenous leukemia myeloid progenitor cells. Proc Natl Acad Sci USA. 1999; 96:10332–10337. PMID: 10468608.
Article15. Koski GK, Schwartz GN, Weng DE, Gress RE, Engels FH, Tsokos M, et al. Calcium ionophore-treated myeloid cells acquire many dendritic cell characteristics independent of prior differentiation state, transformation status, or sensitivity to biologic agents. Blood. 1999; 94:1359–1371. PMID: 10438724.
Article16. Westers TM, Stam AG, Scheper RJ, Regelink JC, Nieuwint AW, Schuurhuis GJ, et al. Rapid generation of antigen-presenting cells from leukaemic blasts in acute myeloid leukaemia. Cancer Immunol Immunother. 2003; 52:17–27. PMID: 12536236.
Article17. Slukvin II, Vodyanik MA, Thomson JA, Gumenyuk ME, Choi KD. Directed differentiation of human embryonic stem cells into functional dendritic cells through the myeloid pathway. J Immunol. 2006; 176:2924–2932. PMID: 16493050.
Article18. Lee JJ, Nam CE, Nam JH, Lee HC, Chung IJ, Park MS, et al. Generation of cytotoxic donor CD8+ T cells against relapsing leukemic cells following allogeneic transplantation by stimulation with leukemic cell- or leukemic lysate pulsed donor cell-derived dendritic cells. Leuk Res. 2004; 28:517–524. PMID: 15068905.
Article19. Choi BH, Kang HK, Park JS, Kim SK, Nguyen Pham TN, Zhu XW, et al. Optimization of the concentration of autologous serum for generation of leukemic dendritic cells from acute myeloid leukemia cells for clinical immunotherapy. J Clin Apher. 2006; 21:233–240. PMID: 17120232.20. Kang HK, Park JS, Kim SK, Choi BH, Nguyen Pham TN, Zhu XW, et al. Down-regulation of cellular VEGF levels induces differentiation of leukemic cells to functional leukemic-dendritic cells in acute myeloid leukemia. Leuk Lymphoma. 2006; 47:2224–2233. PMID: 17071499.21. Davis TA, Saini AA, Blair PJ, Levine BL, Craighead N, Harlan DM, et al. Phorbol esters induce differentiation of human CD34+ hemopoietic progenitors to dendritic cells: evidence for protein kinase C-mediated signaling. J Immunol. 1998; 160:3689–3697. PMID: 9558069.22. Koski GK, Schwartz GN, Weng DE, Czerniecki BJ, Carter C, Gress RE, et al. Calcium mobilization in human myeloid cells results in acquisition of individual dendritic cell-like characteristics through discrete signaling pathways. J Immunol. 1999; 163:82–92. PMID: 10384103.23. Engels FH, Kreisel D, Faries MB, Bedrosian I, Koski GK, Cohen PA, et al. Calcium ionophore activation of chronic myelogenous leukemia progenitor cells into dendritic cells is mediated by calcineurin phosphatase. Leuk Res. 2000; 24:795–804. PMID: 10996197.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Generation of Leukemic Dendritic Cells from Patients with Acute Myelogenous Leukemia
- Mapping of Phorbol Ester and Calcium Ionophore - Responsive Sequence Element in the c - jun Promoter in Activated T Cells
- Generation and Qualification of Functionally Active Leukemia-derived DCs from Malignant Blasts in Acute Leukemia
- Intraparenchymal Myeloid Sarcoma and Subsequent Spinal Myeloid Sarcoma for Acute Myeloblastic Leukemia
- Differentiation induction of dendritic cell phenotypes from human leukemic cell lines