Cancer Res Treat.
2009 Mar;41(1):36-44.
Proapoptotic Ginsenosides Compound K and Rh2 Enhance Fas-induced Cell Death of Human Astrocytoma Cells Through Distinct Apoptotic Signaling Pathways
- Affiliations
-
- 1Laboratory of Computational Cell Biology, Department of Bio and Brain Engineering, KAIST, Daejeon, Korea. cchoi@kaist.ac.kr
- 2Graduate School of Medical Science and Engineering, KAIST, Daejeon, Korea.
- 3KI for the BioCentury, KAIST, Daejeon, Korea.
Abstract
-
PURPOSE: Malignant astrocytomas are among the commonest primary brain tumors and they have a grave prognosis, and so there is an urgent need to develop effective treatment. In this study, we investigated the molecular mechanisms that are responsible for the anti-tumor effect of ginsenosides on human astrocytoma cells.
MATERIALS AND METHODS
We tested 13 different ginsenosides for their anti-tumor effect on human malignant astrocytoma cells in conjunction with Fas stimulation. In addition, the cell signaling pathways were explored by using pharmacological inhibitors and performing immunoblot analysis. DCF-DA staining and antioxidant experiments were performed to investigate the role of reactive oxygen species as one of the apoptosis-inducing mechanisms.
RESULTS
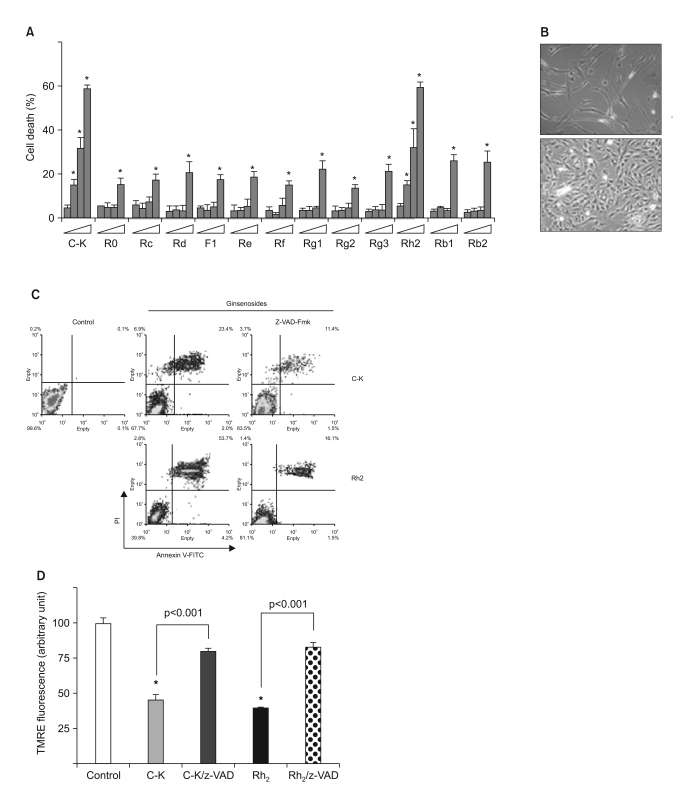
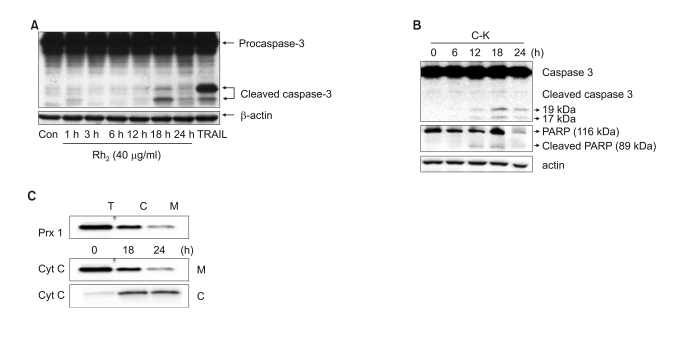
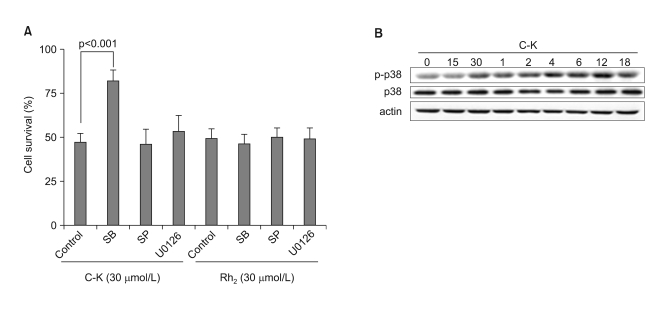
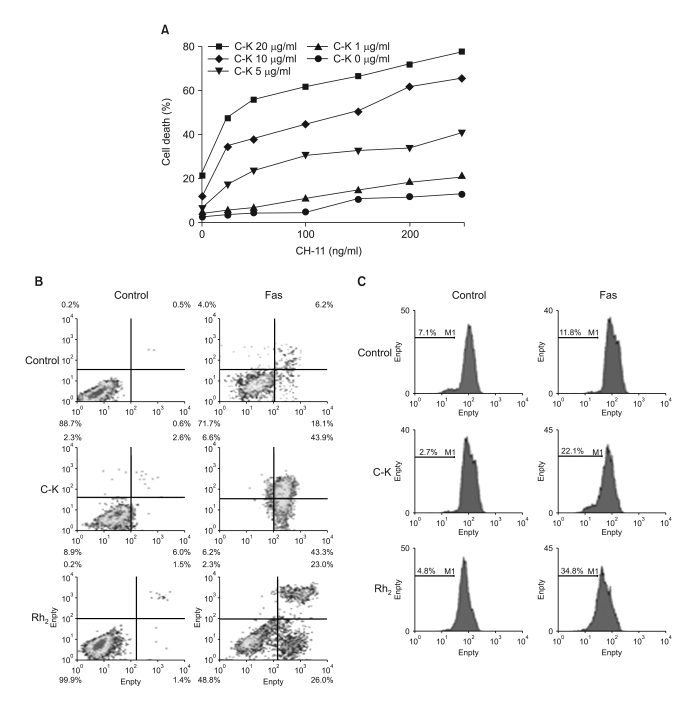
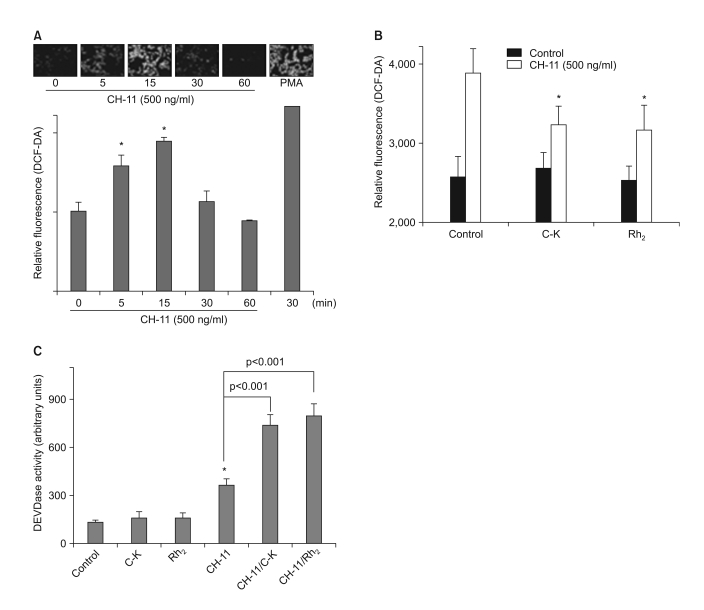
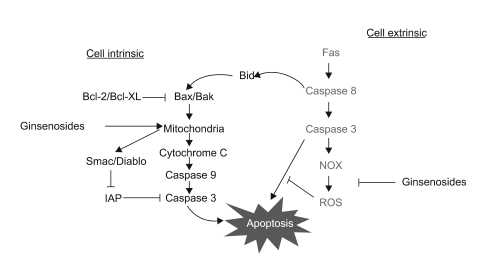
Among the 13 different ginsenoside metabolites, compound K and Rh2 induced apoptotic cell death of the astrocytoma cells in a caspase- and p38 MAPK-dependent manner, yet the same treatment had no cytotoxic effect on the primary cultured human astrocytes. Combined treatment with ginsenosides and Fas ligand showed a synergistic cytotoxic effect, which was mediated by the reduction of intracellular reactive oxygen species.
CONCLUSION
These results suggest that ginsenoside metabolites in combination with Fas ligand may provide a new strategy to treat malignant astrocytomas, which are tumors that are quite resistant to conventional anti-cancer treatment.
Keyword
MeSH Terms
Figure
Reference
-
2. Choi C, Benveniste EN. Fas ligand/Fas system in the brain: regulator of immune and apoptotic responses. Brain Res Brain Res Rev. 2004; 44:65–81. PMID: 14739003.
Article3. Shimada K, Nakamura M, Ishida E, Kishi M, Matsuyoshi S, Konishi N. The molecular mechanism of sensitization to Fas-mediated apoptosis by 2-methoxyestradiol in PC3 prostate cancer cells. Mol Carcinog. 2004; 39:1–9. PMID: 14694442.
Article4. Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998; 17:1675–1687. PMID: 9501089.
Article5. Bae EA, Kim EJ, Park JS, Kim HS, Ryu JH, Kim DH. Ginsenosides Rg3 and Rh2 inhibit the activation of AP-1 and protein kinase A pathway in lipopolysaccharide/interferon-gamma-stimulated BV-2 microglial cells. Planta Med. 2006; 72:627–633. PMID: 16673329.6. Liu Y, Zhang JW, Li W, Ma H, Sun J, Deng MC, et al. Ginsenoside metabolites, rather than naturally occurring ginsenosides, lead to inhibition of human cytochrome P450 enzymes. Toxicol Sci. 2006; 91:356–364. PMID: 16547074.
Article7. Lee HU, Bae EA, Han MJ, Kim NJ, Kim DH. Hepatoprotective effect of ginsenoside Rb1 and compound K on tert-butyl hydroperoxide-induced liver injury. Liver Int. 2005; 25:1069–1073. PMID: 16162168.
Article8. Oh GS, Pae HO, Choi BM, Seo EA, Kim DH, Shin MK, et al. 20(S)-Protopanaxatriol, one of ginsenoside metabolites, inhibits inducible nitric oxide synthase and cyclooxygenase-2 expressions through inactivation of nuclear factor-kappaB in RAW 264.7 macrophages stimulated with lipopolysaccharide. Cancer Lett. 2004; 205:23–29. PMID: 15036657.9. Choi K, Han YH, Choi C. N-Acetyl cysteine and caffeic acid phenethyl ester sensitize astrocytoma cells to Fas-mediated cell death in a redox-dependent manner. Cancer Lett. 2007; 257:79–86. PMID: 17692455.
Article10. Choi C, Kutsch O, Park J, Zhou T, Seol DW, Benveniste EN. Tumor necrosis factor-related apoptosis-inducing ligand induces caspase-dependent interleukin-8 expression and apoptosis in human astroglioma cells. Mol Cell Biol. 2002; 22:724–736. PMID: 11784850.
Article11. Choi C, Park JY, Lee J, Lim JH, Shin EC, Ahn YS, et al. Fas ligand and Fas are expressed constitutively in human astrocytes and the expression increases with IL-1, IL-6, TNF-alpha, or IFN-gamma. J Immunol. 1999; 162:1889–1895. PMID: 9973455.12. Tanaka Y, Nakayamada S, Fujimoto H, Okada Y, Umehara H, Kataoka T, et al. H-Ras/mitogen-activated protein kinase pathway inhibits integrin-mediated adhesion and induces apoptosis in osteoblasts. J Biol Chem. 2002; 277:21446–21452. PMID: 11934900.
Article13. Loveland BE, Johns TG, Mackay IR, Vaillant F, Wang ZX, Hertzog PJ. Validation of the MTT dye assay for enumeration of cells in proliferative and antiproliferative assays. Biochem Int. 1992; 27:501–510. PMID: 1417886.14. Kim JH, Cho SY, Lee JH, Jeong SM, Yoon IS, Lee BH, et al. Neuroprotective effects of ginsenoside Rg3 against homocysteine-induced excitotoxicity in rat hippocampus. Brain Res. 2007; 1136:190–199. PMID: 17239831.
Article15. Choi K, Kim M, Ryu J, Choi C. Ginsenosides compound K and Rh2 inhibit tumor necrosis factor-a-induced activation of the NF-kB and JNK pathways in human astroglial cells. Neurosci Lett. 2007; 421:37–41. PMID: 17548155.16. Choi C, Jeong E, Benveniste EN. Caspase-1 mediates Fas-induced apoptosis and is up-regulated by interferon-γ in human astrocytoma cells. J Neurooncol. 2004; 67:167–176. PMID: 15072464.
Article17. Surh YJ, Na HK, Lee JY, Keum YS. Molecular mechanisms underlying anti-tumor promoting activities of heat-processed Panax ginseng C.A. Meyer. J Korean Med Sci. 2001; 16(Suppl):S38–S41. PMID: 11748375.
Article18. Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007; 26:3100–3112. PMID: 17496909.
Article19. Wang H, Wang Z, Chen J, Wu J. Apoptosis induced by NO via phosphorylation of p38 MAPK that stimulates NF-κB, p53 and caspase-3 activation in rabbit articular chondrocytes. Cell Biol Int. 2007; 31:1027–1035. PMID: 17468017.
Article20. Omuro AM, Faivre S, Raymond E. Lessons learned in the development of targeted therapy for malignant gliomas. Mol Cancer Ther. 2007; 6:1909–1919. PMID: 17620423.
Article21. Clement MV, Stamenkovic I. Superoxide anion is a natural inhibitor of Fas-mediated cell death. EMBO J. 1996; 15:216–225. PMID: 8617197.22. Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006; 312:1882–1883. PMID: 16809515.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation of Fas and FasL mRNA expression in human astrocytoma cell lines by cytokines
- A Study on Apoptotic Signaling Pathway in HL-60 Cells Induced by Radiation
- Effects of Cycloheximide and Dexamethasone on Fas - Mediated Apopthsis in Primary Human Astrocytes
- The Study of the Cytotoxic Mechanism of Cisplatin in HeLa Cells
- Apoptosis of Fas Expressed Target Cells Induced by Cytotoxic T Lymphocytes