Cancer Res Treat.
2009 Jun;41(2):80-86.
Modest Anti-Cancer Activity of a Bile Acid Acylated Heparin Derivative in a PC14PE6 Induced Orthotopic Lung Cancer Model
- Affiliations
-
- 1Medical Nanoelement Development Center, Seoul, Korea.
- 2Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. kpark@skku.edu
- 3Department of Biomedical Engineering, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4College of Pharmacy, Seoul National University, Seoul, Korea.
Abstract
- PURPOSE
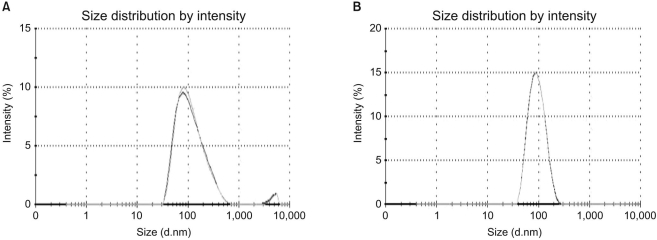
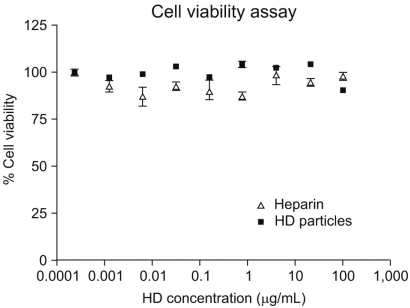
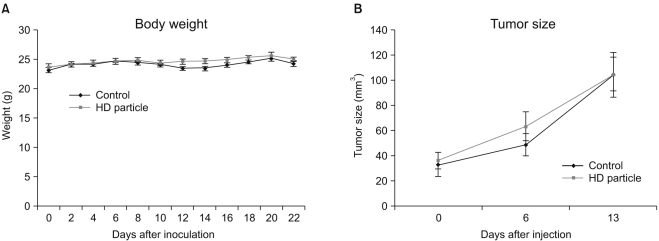
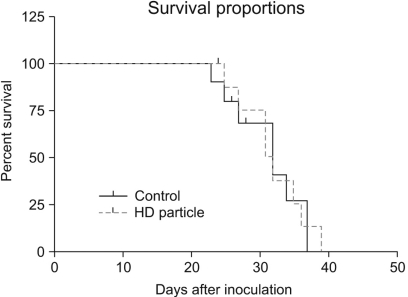
A novel chemically modified heparin derivative, heparin-deoxycholic acid nano-particles, has lower anticoagulant activity, and was recently reported to have significant anti-tumor effects on squamous head and neck cancer cells. Therefore, the aim of this study was to evaluate the anti-tumor effects of heparin-deoxycholic acid nano-particles in a human lung adenocarcinoma cell line. MATERIALS AND METHODS: An orthotopic lung cancer model in 16 mice was developed using intra-thoracic injections of 0.5x10(6) PC14PE6 cells. Ten days after inoculation, the mice were divided into two groups. PBS and Heparin-DOCA particles were injected once a day every 3 days in the tail vein, for a total of 5 injections. The body weight and survival of each mouse were monitored and the tumor size in the lung was measured by SPECT-CT before and after heparin-DOCA nano-particle treatment. RESULTS: IThe HD particles had no significant cytotoxicity when the PC9 cells were treated in vitro. There was no statistical difference in tumor size, body weight and survival between the HD treated and control groups in vivo. Furthermore, there was no difference in the amount of CD31 between tumor tissues in the two study groups. CONCLUSION: HD synthesized with unfractionated heparin had no apparent inhibitory effects on tumor growth in a PC14PE6 cell induced orthotopic lung cancer mouse model. The HD particles did not significantly inhibit tumor-induced angiogenesis at the tumor sites.
Keyword
MeSH Terms
Figure
Reference
-
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108. PMID: 15761078.
Article2. Shin HR, Jung KW, Won YJ, Kong HJ, Yim SH, Sung JH, et al. National cancer Incidence for the year 2002 in Korea. Cancer Res Treat. 2007; 39:139–149.
Article3. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002; 346:92–98. PMID: 11784875.
Article4. Scagliotti GV, De Marinis F, Rinaldi M, Crino L, Gridelli C, Ricci S, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002; 20:4285–4291. PMID: 12409326.
Article5. Kelly K, Crowley J, Bunn PA Jr, Presant CA, Grevstad PK, Moinpour CM, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small-cell lung cancer: a southwest oncology group trial. J Clin Oncol. 2001; 19:3210–3218. PMID: 11432888.
Article6. Smorenburg SM, Van Noorden CJ. The complex effects of heparins on cancer progression and metastasis in experimental studies. Pharmacol Rev. 2001; 53:93–105. PMID: 11171940.7. Klerk CP, Smorenburg SM, Otten HM, Lensing AW, Prins MH, Piovella F, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005; 23:2130–2135. PMID: 15699479.
Article8. Stevenson JL, Choi SH, Varki A. Differential metastasis inhibition by clinically relevant levels of heparins--correlation with selectin inhibition, not antithrombotic activity. Clin Cancer Res. 2005; 11(19 Pt 1):7003–7011. PMID: 16203794.
Article9. Ludwig RJ, Alban S, Bistrian R, Boehncke WH, Kaufmann R, Henschler R, et al. The ability of different forms of heparins to suppress P-selectin function in vitro correlates to their inhibitory capacity on bloodborne metastasis in vivo. Thromb Haemost. 2006; 95:535–540. PMID: 16525583.
Article10. Pumphrey CY, Theus AM, Li S, Parrish RS, Sanderson RD. Neoglycans, carbodiimide-modified glycosaminoglycans: a new class of anticancer agents that inhibit cancer cell proliferation and induce apoptosis. Cancer Res. 2002; 62:3722–3728. PMID: 12097281.11. Lee AY, Rickles FR, Julian JA, Gent M, Baker RI, Bowden C, et al. Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J Clin Oncol. 2005; 23:2123–2129. PMID: 15699480.
Article12. Lundin L, Larsson H, Kreuger J, Kanda S, Lindahl U, Salmivirta M, et al. Selectively desulfated heparin inhibits fibroblast growth factor-induced mitogenicity and angiogenesis. J Biol Chem. 2000; 275:24653–24660. PMID: 10816596.
Article13. Ono K, Ishihara M, Ishikawa K, Ozeki Y, Deguchi H, Sato M, et al. Periodate-treated, non-anticoagulant heparin-carrying polystyrene (NAC-HCPS) affects angiogenesis and inhibits subcutaneous induced tumour growth and metastasis to the lung. Br J Cancer. 2002; 86:1803–1812. PMID: 12087470.
Article14. Park K, Kim YS, Lee GY, Nam JO, Lee SK, Park RW, et al. Antiangiogenic effect of bile acid acylated heparin derivative. Pharm Res. 2007; 24:176–185. PMID: 17109210.
Article15. Cui ZY, Ahn JS, Lee JY, Kim WS, Lim HY, Jeon HJ, et al. Mouse orthotopic lung cancer model induced by PC14PE6. Cancer Res Treat. 2006; 38:234–239.
Article16. Park K, Lee GY, Kim YS, Yu M, Park RW, Kim IS, et al. Heparin-deoxycholic acid chemical conjugate as an anticancer drug carrier and its antitumor activity. J Control Release. 2006; 114:300–306. PMID: 16884806.
Article17. Hong KJ, Choi Y, Lee SC, Lee SY, Song TY, Min BJ, et al. A compact SPECT/CT system for small animal imaging. IEEE Trans Nucl Sci. 2006; 53:2601–2604.
Article18. Park K, Kim K, Kwon IC, Kim SK, Lee S, Lee DY, et al. Preparation and characterization of self-assembled nanoparticles of heparin-deoxycholic acid conjugates. Langmuir. 2004; 20:11726–11731. PMID: 15595804.
Article19. Kakkar AK, Levine MN, Kadziola Z, Lemoine NR, Low V, Patel HK, et al. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS). J Clin Oncol. 2004; 22:1944–1948. PMID: 15143088.
Article20. Niers TM, Klerk CP, DiNisio M, Van Noorden CJ, Buller HR, Reitsma PH, et al. Mechanisms of heparin induced anti-cancer activity in experimental cancer models. Crit Rev Oncol Hematol. 2007; 61:195–207. PMID: 17074500.
Article21. Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci U S A. 2001; 98:3352–3357. PMID: 11248082.
Article22. Park K, Ki Lee S, Hyun Son D, Ah Park S, Kim K, Won Chang H, et al. The attenuation of experimental lung metastasis by a bile acid acylated-heparin derivative. Biomaterials. 2007; 28:2667–2676. PMID: 17335894.
Article23. Lee DY, Park K, Kim SK, Park RW, Kwon IC, Kim SY, et al. Antimetastatic effect of an orally active heparin derivative on experimentally induced metastasis. Clin Cancer Res. 2008; 14:2841–2849. PMID: 18451252.
Article24. Smorenburg SM, Vink R, te Lintelo M, Tigchelaar W, Maas A, Buller HR, et al. In vivo treatment of rats with unfractionated heparin (UFH) or low molecular weight heparin (LMWH) does not affect experimentally induced colon carcinoma metastasis. Clin Exp Metastasis. 1999; 17:451–456. PMID: 10651313.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mouse Orthotopic Lung Cancer Model Induced by PC14PE6
- The Incidence Rate and Severity of Orthotopic Lung Cancer in an Animal Model Depends on the Number of A549 Cells and Transplantation Period
- SP-8356, a (1S)-(-)-Verbenone Derivative, Inhibits the Growth and Motility of Liver Cancer Cells by Regulating NF-κB and ERK Signaling
- Synthetic Bile Acid Derivative HS-1200-induced Apoptosis of Human Osteosarcoma Cells
- Biliary Bile Acid Analysis in the Patients with Bile Duct Cancer