Int J Stem Cells.
2016 May;9(1):107-114. 10.15283/ijsc.2016.9.1.107.
L-carnitine Effectively Induces hTERT Gene Expression of Human Adipose Tissue-derived Mesenchymal Stem Cells Obtained from the Aged Subjects
- Affiliations
-
- 1Department of Clinical Biochemistry, Faculty of Medical Sciences, Tarbiat Modares University, Tabriz, Iran. mesbahna@modares.ac.ir
- 2Department of Biochemistry and Clinical Laboratories, Faculty of Medicine, Tabriz, Iran.
- 3Department of Medical Biotechnology, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
- 4Department of Clinical Sciences, Faculty of Veterinary Medicine, University of Tabriz, Tabriz, Iran.
- KMID: 2164167
- DOI: http://doi.org/10.15283/ijsc.2016.9.1.107
Abstract
- BACKGROUND AND OBJECTIVES
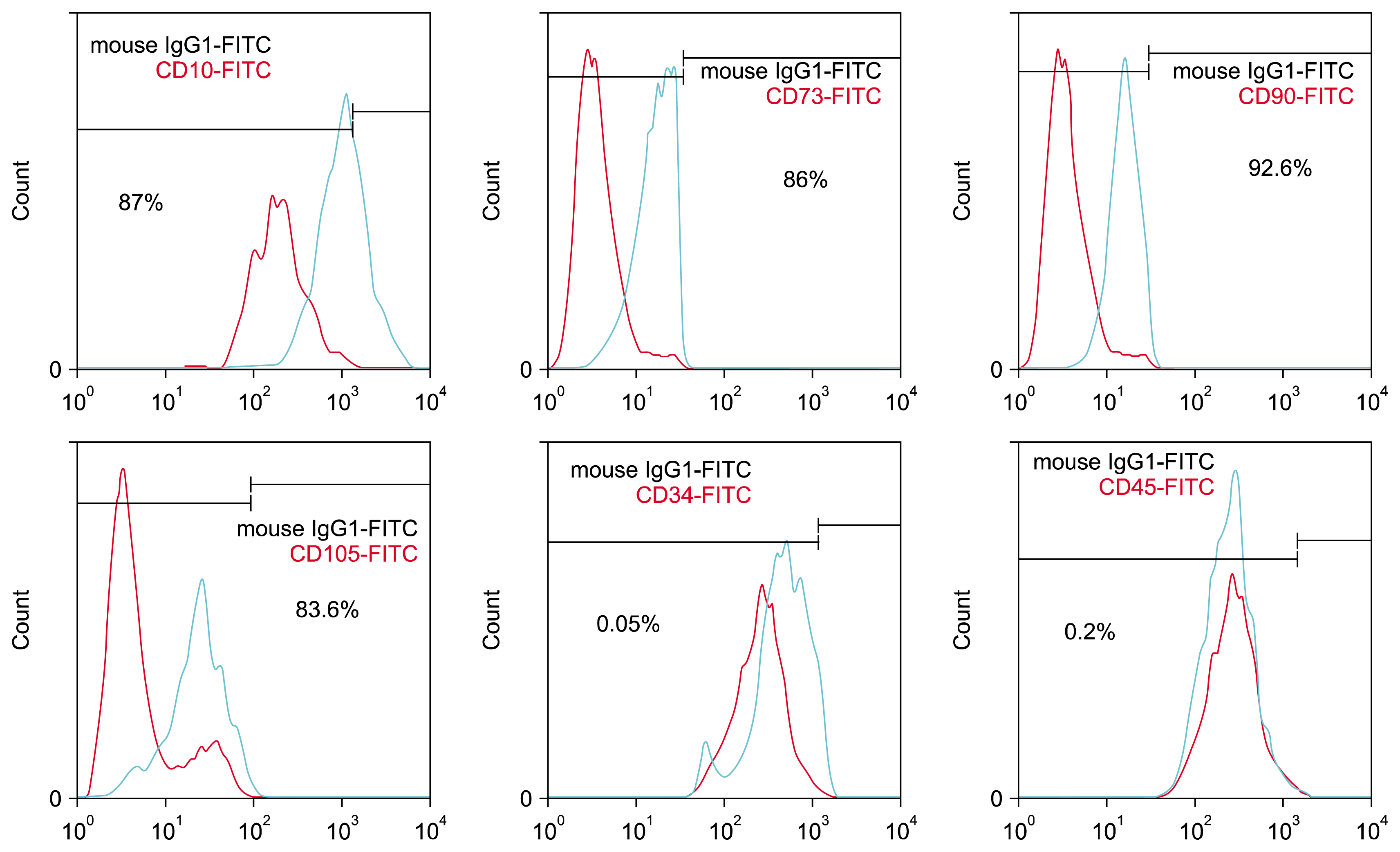
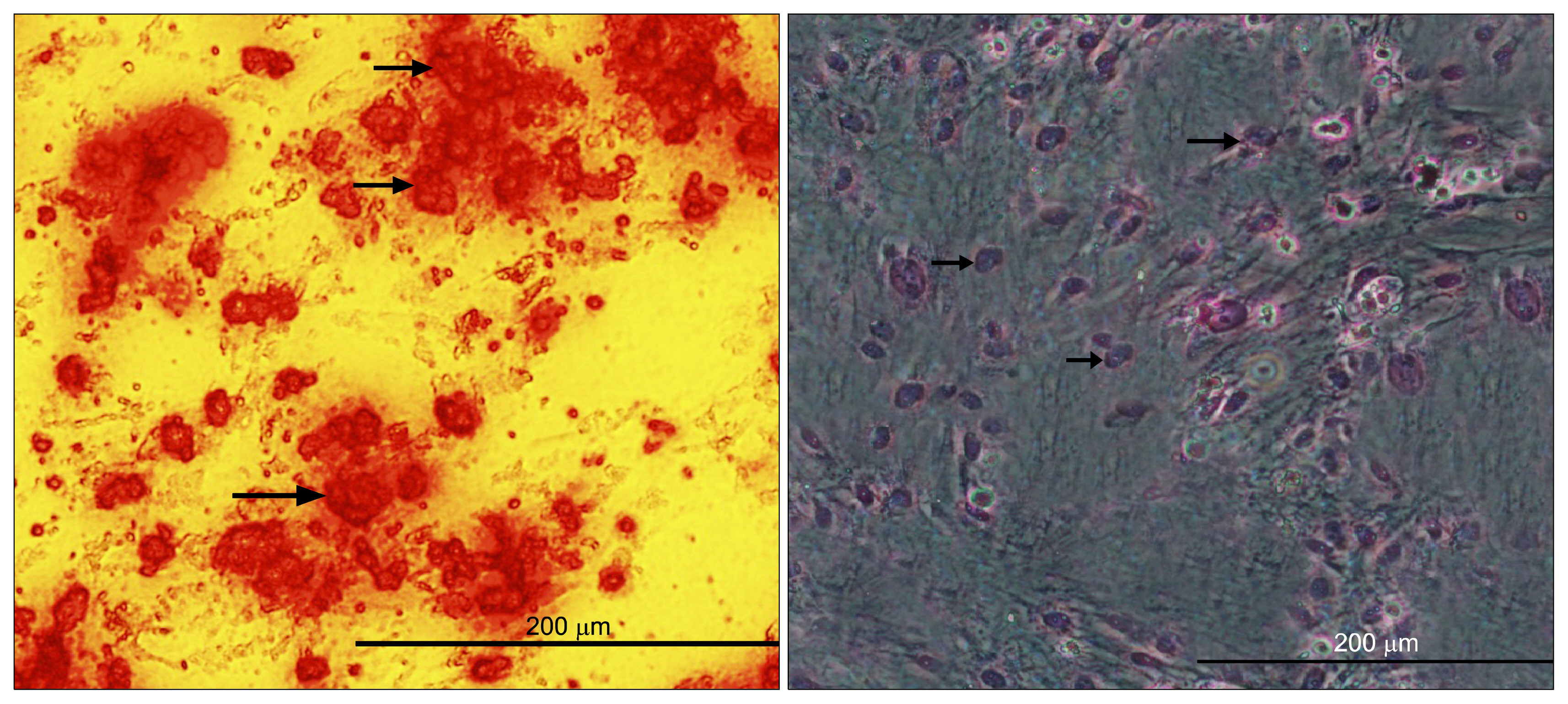
Human mesenchymal stem cells (hMSCs) are attractive candidates for cell therapy and regenerative medicine due to their multipotency and ready availability, but their application can be complicated by the factors such as age of the donors and senescence-associated growth arrest during culture conditions. The latter most likely reflects the fact that aging of hMSCs is associated with a rise in intracellular reactive oxygen species, loss of telomerase activity, decrease in human telomerase reverse transcriptase (hTERT) expression and finally eroded telomere ends. Over-expression of telomerase in hMSCs leads to telomere elongation and may help to maintain replicative life-span of these cells. The aim of this study was to evaluate of the effect of L-carnitine (LC) as an antioxidant on the telomerase gene expression and telomere length in aged adipose tissue-derived hMSCs.
METHODS
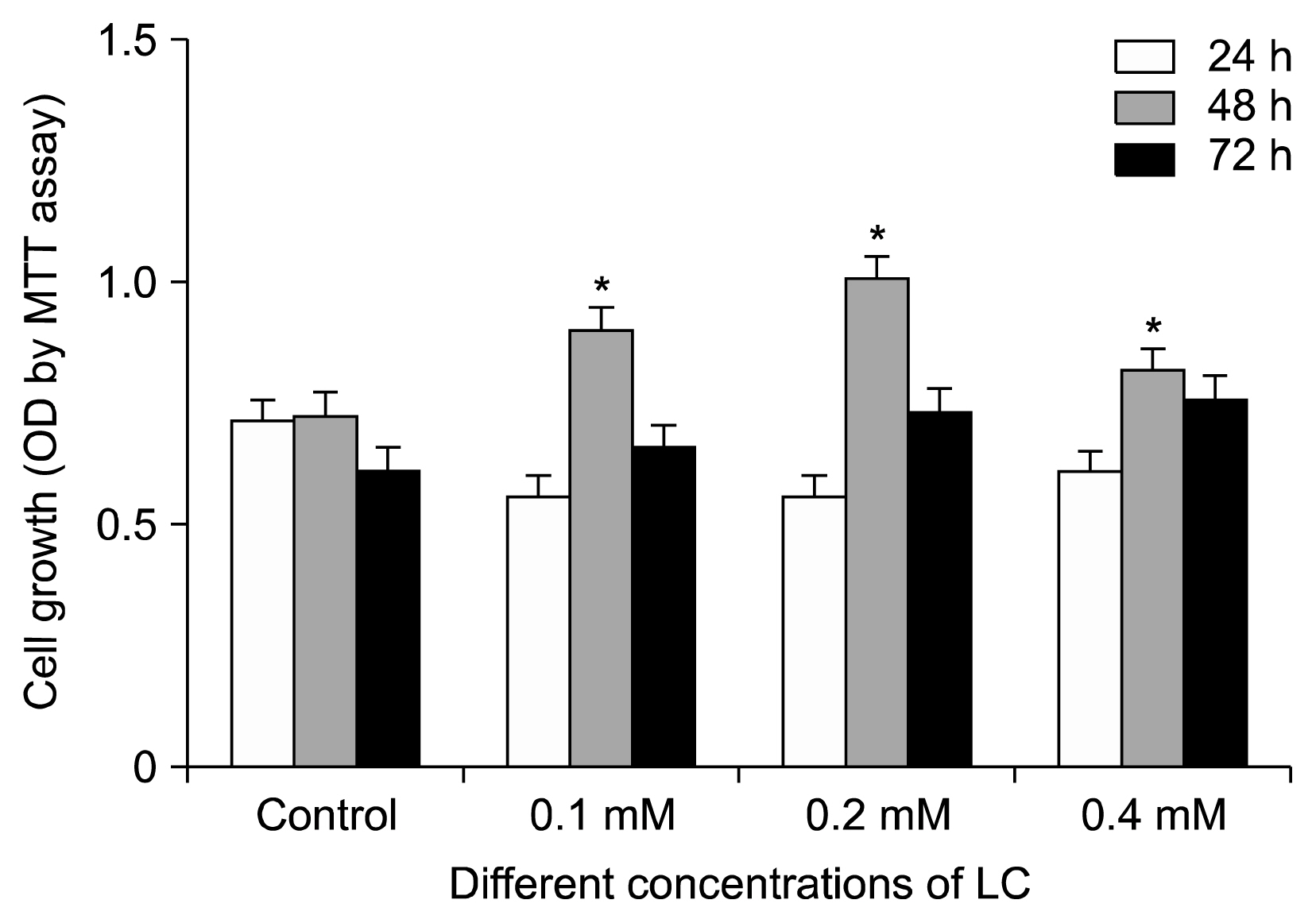
For this purpose, cells were isolated from healthy aged volunteers and their viabilities were assessed by MTT assay. Quantitative gene expression of hTERT and absolute telomere length measurement were also performed by real- time PCR in the absence and presence of different doses of LC (0.1, 0.2 and 0.4 mM).
RESULTS
The results indicated that LC could significantly increase the hTERT gene expression and telomere length, especially in dose of 0.2 mM of LC and in 48 h treatment for the aged adipose tissue-derived hMSCs samples.
CONCLUSION
It seems that LC would be a good candidate to improve the lifespan of the aged adipose tissue-derived hMSCs due to over-expression of telomerase and lengthening of the telomeres.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Misiti S, Nanni S, Fontemaggi G, Cong YS, Wen J, Hirte HW, Piaggio G, Sacchi A, Pontecorvi A, Bacchetti S, Farsetti A. Induction of hTERT expression and telomerase activity by estrogens in human ovary epithelium cells. Mol Cell Biol. 2000; 20:3764–3771. DOI: 10.1128/MCB.20.11.3764-3771.2000. PMID: 10805720. PMCID: 85692.
Article2. Zimmermann S, Voss M, Kaiser S, Kapp U, Waller CF, Martens UM. Lack of telomerase activity in human mesenchymal stem cells. Leukemia. 2003; 17:1146–1149. DOI: 10.1038/sj.leu.2402962. PMID: 12764382.
Article3. Hiyama E, Hiyama K. Telomere and telomerase in stem cells. Br J Cancer. 2007; 96:1020–1024. DOI: 10.1038/sj.bjc.6603671. PMID: 17353922. PMCID: 2360127.
Article4. Li K, Zhu H, Han X, Xing Y. Ectopic hTERT gene expression in human bone marrow mesenchymal stem cell. Life Sci J. 2007; 4:21–24.5. Böcker W, Yin Z, Drosse I, Haasters F, Rossmann O, Wierer M, Popov C, Locher M, Mutschler W, Docheva D, Schieker M. Introducing a single-cell-derived human mesenchymal stem cell line expressing hTERT after lentiviral gene transfer. J Cell Mol Med. 2008; 12:1347–1359. DOI: 10.1111/j.1582-4934.2008.00299.x. PMID: 18318690. PMCID: 3865677.
Article6. Beane OS, Fonseca VC, Cooper LL, Koren G, Darling EM. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS One. 2014; 9:e115963. DOI: 10.1371/journal.pone.0115963. PMID: 25541697. PMCID: 4277426.
Article7. Lu Q, Zhang Y, Elisseeff JH. Carnitine and acetylcarnitine modulate mesenchymal differentiation of adult stem cells. J Tissue Eng Regen Med. 2013; DOI: 10.1002/term.1747. [Epub ahead of print].
Article8. Syslová K, Böhmová A, Mikoška M, Kuzma M, Pelclová D, Kačer P. Multimarker screening of oxidative stress in aging. Oxid Med Cell Longev. 2014; 2014:562860. DOI: 10.1155/2014/562860. PMID: 25147595. PMCID: 4124763.
Article9. Calò LA, Pagnin E, Davis PA, Semplicini A, Nicolai R, Calvani M, Pessina AC. Antioxidant effect of L-carnitine and its short chain esters: relevance for the protection from oxidative stress related cardiovascular damage. Int J Cardiol. 2006; 107:54–60. DOI: 10.1016/j.ijcard.2005.02.053.10. Kitamura Y, Satoh K, Satoh T, Takita M, Matsuura A. Effect of L-carnitine on erythroid colony formation in mouse bone marrow cells. Nephrol Dial Transplant. 2005; 20:981–984. DOI: 10.1093/ndt/gfh758. PMID: 15769817.
Article11. Kobayashi S, Iwamoto M, Kon K, Waki H, Ando S, Tanaka Y. Acetyl-L-carnitine improves aged brain function. Geriatr Gerontol Int. 2010; 10( Suppl 1):S99–S106. DOI: 10.1111/j.1447-0594.2010.00595.x. PMID: 20590847.
Article12. Deyhim MR, Mesbah-Namin SA, Yari F, Taghikhani M, Amirizadeh N. L-carnitine effectively improves the metabolism and quality of platelet concentrates during storage. Ann Hematol. 2015; 94:671–680. DOI: 10.1007/s00277-014-2243-5.
Article13. Hollister L, Gruber N. Drug treatment of Alzheimer’s disease. Effects on caregiver burden and patient quality of life. Drugs Aging. 1996; 8:47–55. DOI: 10.2165/00002512-199608010-00008. PMID: 8785468.14. Bonavita E. Study of the efficacy and tolerability of L-acetylcarnitine therapy in the senile brain. Int J Clin Pharmacol Ther Toxicol. 1986; 24:511–516. PMID: 3781687.15. Musicco C, Capelli V, Pesce V, Timperio AM, Calvani M, Mosconi L, Cantatore P, Gadaleta MN. Rat liver mitochondrial proteome: changes associated with aging and acetyl-L-carnitine treatment. J Proteomics. 2011; 74:2536–2547. DOI: 10.1016/j.jprot.2011.05.041. PMID: 21672642.
Article16. Sener G, Paskaloğlu K, Satiroglu H, Alican I, Kaçmaz A, Sakarcan A. L-carnitine ameliorates oxidative damage due to chronic renal failure in rats. J Cardiovasc Pharmacol. 2004; 43:698–705. DOI: 10.1097/00005344-200405000-00013. PMID: 15071358.
Article17. Gülçin I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006; 78:803–811. DOI: 10.1016/j.lfs.2005.05.103.
Article18. Thangasamy T, Jeyakumar P, Sittadjody S, Joyee AG, Chinnakannu P. L-carnitine mediates protection against DNA damage in lymphocytes of aged rats. Biogerontology. 2009; 10:163–172. DOI: 10.1007/s10522-008-9159-1.
Article19. Xiao C, Zhou H, Liu G, Zhang P, Fu Y, Gu P, Hou H, Tang T, Fan X. Bone marrow stromal cells with a combined expression of BMP-2 and VEGF-165 enhanced bone regeneration. Biomed Mater. 2011; 6:015013. DOI: 10.1088/1748-6041/6/1/015013. PMID: 21252414.
Article20. Li L, Guo Y, Zhai H, Yin Y, Zhang J, Chen H, Wang L, Li N, Liu R, Xia Y. Aging increases the susceptivity of MSCs to reactive oxygen species and impairs their therapeutic potency for myocardial infarction. PLoS One. 2014; 9:e111850. DOI: 10.1371/journal.pone.0111850. PMID: 25393016. PMCID: 4230939.
Article21. Parsch D, Fellenberg J, Brümmendorf TH, Eschlbeck AM, Richter W. Telomere length and telomerase activity during expansion and differentiation of human mesenchymal stem cells and chondrocytes. J Mol Med (Berl). 2004; 82:49–55. DOI: 10.1007/s00109-003-0506-z.
Article22. Stolzing A, Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell. 2006; 5:213–224. DOI: 10.1111/j.1474-9726.2006.00213.x. PMID: 16842494.
Article23. Sharpless NE, DePinho RA. Telomeres, stem cells, senescence, and cancer. J Clin Invest. 2004; 113:160–168. DOI: 10.1172/JCI20761. PMID: 14722605. PMCID: 311439.
Article24. Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004; 22:675–682. DOI: 10.1634/stemcells.22-5-675. PMID: 15342932.
Article25. Fehrer C, Lepperdinger G. Mesenchymal stem cell aging. Exp Gerontol. 2005; 40:926–930. DOI: 10.1016/j.exger.2005.07.006. PMID: 16125890.
Article26. Britt-Compton B, Wyllie F, Rowson J, Capper R, Jones RE, Baird DM. Telomere dynamics during replicative senescence are not directly modulated by conditions of oxidative stress in IMR90 fibroblast cells. Biogerontology. 2009; 10:683–693. DOI: 10.1007/s10522-009-9216-4. PMID: 19214769.
Article27. Voghel G, Thorin-Trescases N, Mamarbachi AM, Villeneuve L, Mallette FA, Ferbeyre G, Farhat N, Perrault LP, Carrier M, Thorin E. Endogenous oxidative stress prevents telomerase-dependent immortalization of human endothelial cells. Mech Ageing Dev. 2010; 131:354–363. DOI: 10.1016/j.mad.2010.04.004. PMID: 20399802. PMCID: 3700881.
Article28. Abou-Zeid L, Baraka HN. Combating oxidative stress as a hallmark of cancer and aging: Computational modeling and synthesis of phenylene diamine analogs as potential antioxidant. Saudi Pharm J. 2014; 22:264–272. DOI: 10.1016/j.jsps.2013.07.009. PMID: 25061412. PMCID: 4099563.
Article29. Huang H, Liu N, Guo H, Liao S, Li X, Yang C, Liu S, Song W, Liu C, Guan L, Li B, Xu L, Zhang C, Wang X, Dou QP, Liu J. L-carnitine is an endogenous HDAC inhibitor selectively inhibiting cancer cell growth in vivo and in vitro. PLoS One. 2012; 7:e49062. DOI: 10.1371/journal.pone.0049062. PMID: 23139833. PMCID: 3489732.
Article30. O’Callaghan NJ, Fenech M. A quantitative PCR method for measuring absolute telomere length. Biol Proced Online. 2011; 13:3. DOI: 10.1186/1480-9222-13-3. PMCID: 3047434.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison with human amniotic membrane- and adipose tissue-derived mesenchymal stem cells
- Adipose-derived stem cells: characterization and clinical application
- The Rapid Establishment of Human Clonal Adipose Derived Stem Cell (hADSC) Lines with Aspirated Adipose Tissue
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Adipose Tissue - Adequate, Accessible Regenerative Material