J Korean Med Sci.
2013 Jun;28(6):939-945. 10.3346/jkms.2013.28.6.939.
Resveratrol Has Anabolic Effects on Disc Degeneration in a Rabbit Model
- Affiliations
-
- 1Department of Neurosurgery, Sungkyunkwan University School of Medicine, Kangbuk Samsung Hospital, Seoul, Korea. neuriac@skku.edu
- KMID: 2158045
- DOI: http://doi.org/10.3346/jkms.2013.28.6.939
Abstract
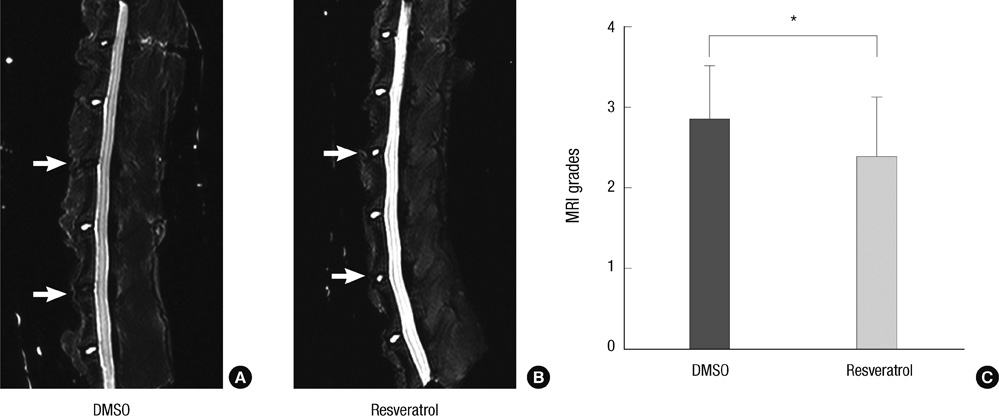
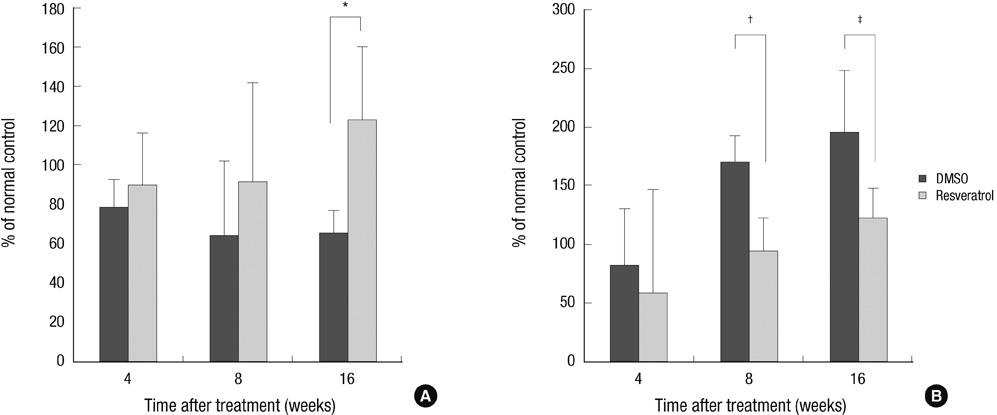
- This study was done to evaluate whether injections of resveratrol, a natural compound found in the skin of grapes, had anabolic effects on degenerated intervertebral discs in a rabbit model. Two non-continuous lumbar discs were punctured in rabbits to induce disc degeneration. Four weeks and 6 weeks after puncture, the rabbits were treated by injections with dimethylsulfoxide (DMSO) or resveratrol. At 4, 8, and 16 weeks after initial injection, rabbits were sacrificed and the spine was extracted for magnetic resonance image (MRI), mRNA expression, and histological staining. Resveratrol treatment resulted in stronger signal intensity in T2-weighted images. MRI grade showed significantly lower in the resveratrol group than the DMSO group (P = 0.039). In the resveratrol group, aggrecan gene expression was significantly increased than that in the DMSO group at 16 weeks after injection (P = 0.027). MMP-13 mRNA levels in the resveratrol group were significantly decreased than those in the DMSO group at 8 and 16 weeks (P = 0.006 and P = 0.048, respectively). In hematoxylin and eosin stain, resveratrol-treated discs showed the features of regeneration. Histologic grade revealed improvement in resveratrol-treated discs, compared with DMSO-treated discs (P = 0.024). These anabolic effects on degenerated discs indicate that resveratrol is a promising candidate for treatment of degenerative disc disease.
MeSH Terms
-
Aggrecans/genetics/metabolism
Anabolic Agents/*administration & dosage
Animals
Disease Models, Animal
Drug Administration Schedule
Intervertebral Disc Degeneration/*drug therapy/pathology
Magnetic Resonance Imaging
Matrix Metalloproteinase 13/genetics/metabolism
RNA, Messenger/metabolism
Rabbits
Spine/radiography
Stilbenes/*administration & dosage
Aggrecans
Anabolic Agents
RNA, Messenger
Stilbenes
Matrix Metalloproteinase 13
Figure
Reference
-
1. Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford). 2009; 48:5–10.2. Freemont TJ, LeMaitre C, Watkins A, Hoyland JA. Degeneration of intervertebral discs: current understanding of cellular and molecular events, and implications for novel therapies. Expert Rev Mol Med. 2001; 2001:1–10.3. Chujo T, An HS, Akeda K, Miyamoto K, Muehleman C, Attawia M, Andersson G, Masuda K. Effects of growth differentiation factor-5 on the intervertebral disc: in vitro bovine study and in vivo rabbit disc degeneration model study. Spine (Phila Pa 1976). 2006; 31:2909–2917.4. Hohaus C, Ganey TM, Minkus Y, Meisel HJ. Cell transplantation in lumbar spine disc degeneration disease. Eur Spine J. 2008; 17:492–503.5. Kandel R, Roberts S, Urban JP. Tissue engineering and the intervertebral disc: the challenges. Eur Spine J. 2008; 17:480–491.6. Kwon YJ, Lee JW, Moon EJ, Chung YG, Kim OS, Kim HJ. Anabolic effects of Peniel 2000, a peptide that regulates TGF-β1 signaling on intervertebral disc degeneration. Spine (Phila Pa 1976). 2013; 38:E49–E58.7. Masuda K, Imai Y, Okuma M, Muehleman C, Nakagawa K, Akeda K, Thonar E, Andersson G, An HS. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine (Phila Pa 1976). 2006; 31:742–754.8. Moon SH, Gilbertson LG, Nishida K, Knaub M, Muzzonigro T, Robbins PD, Evans CH, Kang JD. Human intervertebral disc cells are genetically modifiable by adenovirus-mediated gene transfer: implications for the clinical management of intervertebral disc disorders. Spine (Phila Pa 1976). 2000; 25:2573–2579.9. Estrov Z, Shishodia S, Faderl S, Harris D, Van Q, Kantarjian HM, Talpaz M, Aggarwal BB. Resveratrol blocks interleukin-1beta-induced activation of the nuclear transcription factor NF-kappaB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood. 2003; 102:987–995.10. Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000; 164:6509–6519.11. Sun C, Hu Y, Liu X, Wu T, Wang Y, He W, Wei W. Resveratrol downregulates the constitutional activation of nuclear factor-kappaB in multiple myeloma cells, leading to suppression of proliferation and invasion, arrest of cell cycle, and induction of apoptosis. Cancer Genet Cytogenet. 2006; 165:9–19.12. Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxford). 2008; 47:809–814.13. Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005; 7:R732–R745.14. Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007; 9:R77.15. Li X, Phillips FM, An HS, Ellman M, Thonar EJ, Wu W, Park D, Im HJ. The action of resveratrol, a phytoestrogen found in grapes, on the intervertebral disc. Spine (Phila Pa 1976). 2008; 33:2586–2595.16. Wuertz K, Quero L, Sekiguchi M, Klawitter M, Nerlich A, Konno S, Kikuchi S, Boos N. The red wine polyphenol resveratrol shows promising potential for the treatment of nucleus pulposus–mediated pain in vitro and in vivo. Spine (Phila Pa 1976). 2011; 36:E1373–E1384.17. Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, Andersson GB, An HS. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine (Phila Pa 1976). 2005; 30:5–14.18. Sobajima S, Kompel JF, Kim JS, Wallach CJ, Robertson DD, Vogt MT, Kang JD, Gilbertson LG. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine (Phila Pa 1976). 2005; 30:15–24.19. Csaki C, Keshishzadeh N, Fischer K, Shakibaei M. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem Pharmacol. 2008; 75:677–687.20. Shakibaei M, Csaki C, Nebrich S, Mobasheri A. Resveratrol suppresses interleukin-1beta-induced inflammatory signaling and apoptosis in human articular chondrocytes: potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem Pharmacol. 2008; 76:1426–1439.21. Makarov SS. NF-kappa B in rheumatoid arthritis: a pivotal regulator of inflammation, hyperplasia, and tissue destruction. Arthritis Res. 2001; 3:200–206.22. Miagkov AV, Kovalenko DV, Brown CE, Didsbury JR, Cogswell JP, Stimpson SA, Baldwin AS, Makarov SS. NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci U S A. 1998; 95:13859–13864.23. Wehling N, Palmer GD, Pilapil C, Liu F, Wells JW, Müller PE, Evans CH, Porter RM. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009; 60:801–812.24. Subbaramaiah K, Chung WJ, Michaluart P, Telang N, Tanabe T, Inoue H, Jang M, Pezzuto JM, Dannenberg AJ. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J Biol Chem. 1998; 273:21875–21882.25. Elmali N, Esenkaya I, Harma A, Ertem K, Turkoz Y, Mizrak B. Effect of resveratrol in experimental osteoarthritis in rabbits. Inflamm Res. 2005; 54:158–162.26. Urban JP, McMullin JF. Swelling pressure of the inervertebral disc: influence of proteoglycan and collagen contents. Biorheology. 1985; 22:145–157.27. Sobajima S, Shimer AL, Chadderdon RC, Kompel JF, Kim JS, Gilbertson LG, Kang JD. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005; 5:14–23.28. Anderson DG, Izzo MW, Hall DJ, Vaccaro AR, Hilibrand A, Arnold W, Tuan RS, Albert TJ. Comparative gene expression profiling of normal and degenerative discs: analysis of a rabbit annular laceration model. Spine (Phila Pa 1976). 2002; 27:1291–1296.29. Omlor GW, Lorenz H, Engelleiter K, Richter W, Carstens C, Kroeber MW, Guehring T. Changes in gene expression and protein distribution at different stages of mechanically induced disc degeneration: an in vivo study on the New Zealand white rabbit. J Orthop Res. 2006; 24:385–392.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Grape seed extract inhibits nucleus pulposus cell apoptosis and attenuates annular puncture induced intervertebral disc degeneration in rabbit model
- Histological Changes after Intradiscal Steroid Injection to the Intervertebral Disc in Disc Injury Rabbit Model
- Biochemical Factors of Intervertebral Disc Degeneration: Implications for Disc Regeneration
- Intradiscal Gene Therapy: Therapeutic Implications in Degenerative Disc Disease
- Rabbit Model for in vivo Study of Intervertebral Disc Degeneration and Regeneration