J Korean Med Sci.
2012 Dec;27(12):1472-1478. 10.3346/jkms.2012.27.12.1472.
Preventive Effect of Korean Red Ginseng for Acute Respiratory Illness: A Randomized and Double-Blind Clinical Trial
- Affiliations
-
- 1Department of Internal Medicine, Chonbuk National University Medical School, Jeonju, Korea. lcsmd@jbnu.ac.kr
- 2Department of Preventive Medicine, Chonbuk National University Medical School, Jeonju, Korea.
- 3Research Institute of Clinical Medicine of Chonbuk National University and Chonbuk National University Hospital, Jeonju, Korea.
- 4Clinical Trial Center for Functional Foods, Chonbuk National University Hospital, Jeonju, Korea.
- 5Institute of Jinan Red Ginseng, Jinan, Korea.
- 6R & D Center of CheonJiYang Co., Ltd., Seoul, Korea.
- 7Korea Food Research Institute, Seongnam, Korea.
- 8Healthcare Claims and Management, Jeonju, Korea.
- KMID: 2157969
- DOI: http://doi.org/10.3346/jkms.2012.27.12.1472
Abstract
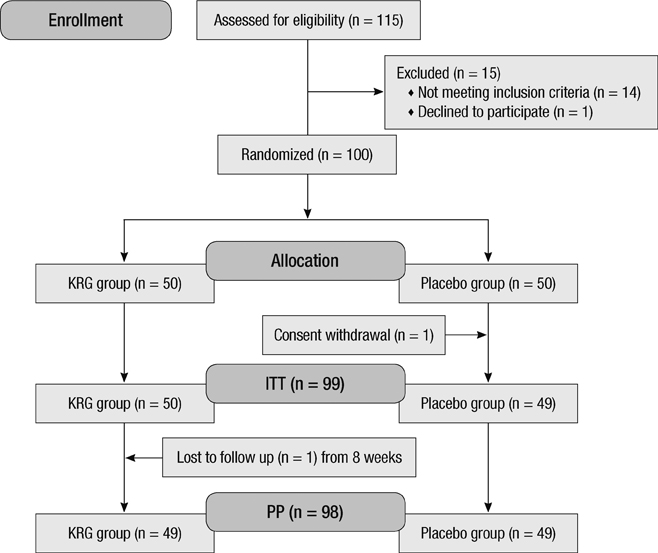
- Korean Red Ginseng (KRG) is a functional food and has been well known for keeping good health due to its anti-fatigue and immunomodulating activities. However, there is no data on Korean red ginseng for its preventive activity against acute respiratory illness (ARI). The study was conducted in a randomized, double-blinded, placebo-controlled trial in healthy volunteers (Clinical Trial Number: NCT01478009). Our primary efficacy end point was the number of ARI reported and secondary efficacy end point was severity of symptoms, number of symptoms, and duration of ARI. A total of 100 volunteers were enrolled in the study. Fewer subjects in the KRG group reported contracting at least 1 ARI than in the placebo group (12 [24.5%] vs 22 [44.9%], P = 0.034), the difference was statistically significant between the two groups. The symptom duration of the subjects who experienced the ARI, was similar between the two groups (KRG vs placebo; 5.2 +/- 2.3 vs 6.3 +/- 5.0, P = 0.475). The symptom scores were low tendency in KRG group (KRG vs placebo; 9.5 +/- 4.5 vs 17.6 +/- 23.1, P = 0.241). The study suggests that KRG may be effective in protecting subjects from contracting ARI, and may have the tendency to decrease the duration and scores of ARI symptoms.
Keyword
MeSH Terms
Figure
Reference
-
1. Turner RB. Mandell GL, Bennett JE, Dolin R, editors. The common cold. Principles and practice of infectious disease. 2010. Volume 1:7th ed. London: Chruchill Livingstone/Elsevier;809–813.2. Rubin MA, Sande MA. Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. Pharyngitis, sinusitis, otitis, and other upper respiratory tract infectiions. Harrison's principles of internal edicine. 2008. 17th ed. New York: McGraw-Hill;205–214.3. Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003. 289:179–186.4. Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, Iskander JK, Wortley PM, Shay DK, Bresee JS, et al. Prevention and control of influenza with: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010. 59:1–62.5. Eliason BC, Kruger J, Mark D, Rasmann DN. Dietary supplement users: demographics, product use, and medical system interaction. J Am Board Fam Pract. 1997. 10:265–271.6. Yale SH, Liu K. Echinacea purpurea therapy for the treatment of the common cold: a randomized, double-blind, placebo-controlled clinical trial. Arch Intern Med. 2004. 164:1237–1241.7. Bae EA, Hyun YJ, Choo MK, Oh JK, Ryu JH, Kim DH. Protective effect of fermented red ginseng on a transient focal ischemic rats. Arch Pharm Res. 2004. 27:1136–1140.8. Scaglione F, Cattaneo G, Alessandria M, Cogo R. Efficacy and safety of the standardised Ginseng extract G115 for potentiating vaccination against the influenza syndrome and protection against the common cold. Drugs Exp Clin Res. 1996. 22:65–72.9. Yun TK, Lee YS, Lee YH, Kim SI, Yun HY. Anticarcinogenic effect of Panax ginseng C.A. Meyer and identification of active compounds. J Korean Med Sci. 2001. 16:S6–S18.10. Song Z, Johansen HK, Faber V, Moser C, Kharazmi A, Rygaard J, Høiby N. Ginseng treatment reduces bacterial load and lung pathology in chronic Pseudomonas aeruginosa pneumonia in rats. Antimicrob Agents Chemother. 1997. 41:961–964.11. McElhaney JE, Gravenstein S, Cole SK, Davidson E, O'neill D, Petitjean S, Rumble B, Shan JJ. A placebo-controlled trial of a proprietary extract of North American ginseng (CVT-E002) to prevent acute respiratory illness in institutionalized older adults. J Am Geriatr Soc. 2004. 52:13–19.12. Predy GN, Goel V, Lovlin R, Donner A, Stitt L, Basu TK. Efficacy of an extract of North American ginseng containing poly-furanosyl-pyranosylsaccharides for preventing upper respiratory tract infections: a randomized controlled trial. CMAJ. 2005. 173:1043–1048.13. McElhaney JE, Goel V, Toane B, Hooten J, Shan JJ. Efficacy of COLD-fX in the prevention of respiratory symptoms in community-dwelling adults: a randomized, double-blinded, placebo controlled trial. J Altern Complement Med. 2006. 12:153–157.14. Kim JY, Kim HJ, Kim HJ. Effects of oral administration of Korean red ginseng on influenza A (H1N1) virus infection. J Ginseng Res. 2011. 35:104–110.15. Quan FS, Compans RW, Cho YK, Kang SM. Ginseng and Salviae herbs play a role as immune activators and modulate immune responses during influenza virus infection. Vaccine. 2007. 25:272–282.16. Wang M, Guilbert LJ, Ling L, Li J, Wu Y, Xu S, Pang P, Shan JJ. Immunomodulating activity of CVT-E002, a proprietary extract from North American ginseng (Panax quinquefolium). J Pharm Pharmacol. 2001. 53:1515–1523.17. Caso Marasco A, Vargas Ruiz R, Salas Villagomez A, Begoña Infante C. Double-blind study of a multivitamin complex supplemented with ginseng extract. Drugs Exp Clin Res. 1996. 22:323–329.18. Ha TS, Choi JY, Park HY, Lee JS. Ginseng total saponin improves podocyte hyperpermeability induced by high glucose and advanced glycosylation endproducts. J Korean Med Sci. 2011. 26:1316–1321.19. Kim JY, Germolec DR, Luster MI. Panax ginseng as a potential immunomodulator: studies in mice. Immunopharmacol Immunotoxicol. 1990. 12:257–276.20. Hu S, Concha C, Johannisson A, Meglia G, Waller KP. Effect of subcutaneous injection of ginseng on cows with subclinical Staphylococcus aureus mastitis. J Vet Med B Infect Dis Vet Public Health. 2001. 48:519–528.21. Luo YM, Cheng XJ, Yuan WX. Effects of ginseng root saponins and ginsenoside Rb1 on immunity in cold water swim stress mice and rats. Zhongguo Yao Li Xue Bao. 1993. 14:401–404.22. Sumiyoshi M, Sakanaka M, Kimura Y. Effects of Red Ginseng extract on allergic reactions to food in Balb/c mice. J Ethnopharmacol. 2010. 132:206–212.23. Block KI, Mead MN. Immune system effects of echinacea, ginseng, and astragalus: a review. Integr Cancer Ther. 2003. 2:247–267.24. Keum YS, Park KK, Lee JM, Chun KS, Park JH, Lee SK, Kwon H, Surh YJ. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett. 2000. 150:41–48.25. Kaneko H, Nakanishi K. Proof of the mysterious efficacy of ginseng: basic and clinical trials: clinical effects of medical ginseng, Korean red ginseng: specifically, its anti-stress action for prevention of disease. J Pharmacol Sci. 2004. 95:158–162.26. Nguyen A, Slavik V. COLD-fX. Can Fam Physician. 2007. 53:481–482.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Study on Chemoprevention of Hepatocellular Carcinoma by Ginseng: An Introduction to the Protocol
- Effect of Korean Red Ginseng on Stress Responses and beta-Adrenergic Receptor Function in a Normal Population
- Effect of Korean Red Ginseng on Sleep: A Randomized, Placebo-Controlled Trial
- Effects of Korean White Ginseng (Panax Ginseng C.A. Meyer) on Vascular and Glycemic Health in Type 2 Diabetes: Results of a Randomized, Double Blind, Placebo-controlled, Multiple-crossover, Acute Dose Escalation Trial
- Effects of Korean Red Ginseng on Cardiovascular Risks in Subjects with Metabolic Syndrome: a Double-blind Randomized Controlled Study