J Korean Med Sci.
2006 Aug;21(4):733-738. 10.3346/jkms.2006.21.4.733.
Upregulation of Proinflammatory Cytokines in the Fetal Brain of the Gaucher Mouse
- Affiliations
-
- 1Department of Biomedical Sciences, National Institute of Health, Korea.
- 2Department of Biochemistry, College of Medicine, Ewha Womans University, Seoul, Korea. jungsc@ewha.ac.kr
- KMID: 2157830
- DOI: http://doi.org/10.3346/jkms.2006.21.4.733
Abstract
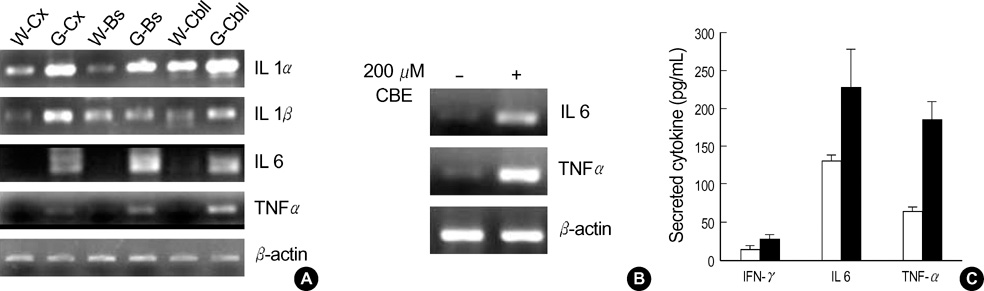
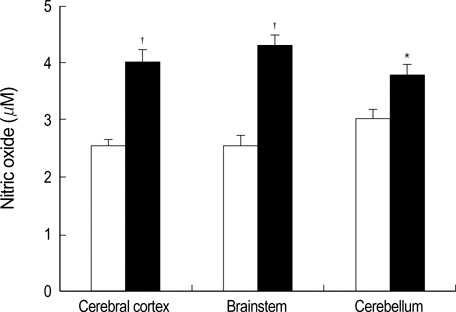
- Gaucher disease is caused by a deficiency of glucocerebrosidase. Patients with Gaucher disease are divided into three major phenotypes: chronic nonneuronopathic, acute neuronopathic, and chronic neuronopathic, based on symptoms of the nervous system, the severity of symptoms, and the age of disease onset. The characteristics of patients with acute neuronopathic- and chronic neuronopathic-type Gaucher disease include oculomotor abnormalities, bulbar signs, limb rigidity, seizures and occasional choreoathetoid movements, and neuronal loss. However, the mechanisms leading to the neurodegeneration of this disorder remain unknown. To investigate brain dysfunction in Gaucher disease, we studied the possible role of inflammation in neurodegeneration during development of Gaucher disease in a mouse model. Elevated levels of the proinflammatory cytokines, IL-1alpha, IL-1beta, IL-6, and TNF-alpha, were detected in the fetal brains of Gaucher mice. Moreover, the levels of secreted nitric oxide and reactive oxygen species in the brains of Gaucher mice were higher than in wild-type mice. Thus, accumulated glucocerebroside or glucosylsphingosine, caused by glucocerebrosidase deficiency, may mediate brain inflammation in the Gaucher mouse via the elevation of proinflammatory cytokines, nitric oxide, and reactive oxygen species.
Keyword
MeSH Terms
-
Up-Regulation/genetics
Tumor Necrosis Factor-alpha/genetics/secretion
Reverse Transcriptase Polymerase Chain Reaction
Reactive Oxygen Species/metabolism
RNA, Messenger/genetics/metabolism
Nitric Oxide/metabolism
Microglia/cytology/metabolism
Mice, Knockout
Mice, Inbred ICR
Mice, Inbred C57BL
Mice
Interleukin-6/genetics/secretion
Interleukin-1/genetics/secretion
Inflammation/immunology
Glucosylceramidase/genetics
Gaucher Disease/*genetics/metabolism/pathology
Cytokines/*genetics/immunology/secretion
Cells, Cultured
Brain/embryology/*metabolism/pathology
Animals
Figure
Reference
-
1. Beutler E, Grabowski GA. Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Gaucher disease. The Metabolic and Molecular Bases of Inherited Disease. 2001. New York: McGraw-Hill;3635–3668.2. Brady RO, Kanfer JN, Bradley RM, Shapiro D. Demonstration of a deficiency of glucocerebroside-cleaving enzyme in Gaucher's disease. J Clin Invest. 1966. 45:1112–1115.
Article3. Brady RO, Barton NW, Grabowski GA. The role of neurogenetics in Gaucher disease. Arch Neurol. 1993. 50:1212–1224.
Article4. Hong YB, Kim EY, Jung SC. Down regulation of Bcl-2 in the fetal brain of Gaucher disease mouse model: a possible role in the neuronal loss. J Hum Genet. 2004. 49:349–354.5. Barak V, Acker M, Nisman B, Kalickman I, Abrahamov A, Zimran A, Yatziv S. Cytokines in Gaucher's disease. Eur Cytokine Netw. 1999. 10:205–210.6. Lichtenstein M, Zimran A, Horowitz M. Cytokine mRNA in Gaucher disease. Blood Cell Mol Dis. 1997. 23:395–401.
Article7. Mizukami H, Mi Y, Wada R, Kono M, Yamashita T, Liu Y, Werth N, Sandhoff R, Sandhoff K, Proia RL. Systemic inflammation in glucocerebrosidase-deficient mice with minimal glucosylceramide storage. J Clin Invest. 2002. 109:1215–1221.
Article8. Baudry M, Yao Y, Simmons D, Liu J, Bi X. Postnatal development of inflammation in a murine model of Niemann-Pick type C disease: immunohistochemical observations of microglia and astroglia. Exp Neurol. 2003. 184:887–903.
Article9. Jeyakumar M, Thomas R, Elliot-Smith E, Smith DA, van der Spoel AC, d'Azzo A, Perry VH, Butters TD, Dwek RA, Platt FM. Central nervous system inflammation is a hallmark of pathogenesis in mouse models of GM1 and GM2 gangliosidosis. Brain. 2003. 126:974–987.
Article10. Wu YP, Proia RL. Deletion of macrophage-inflammatory protein 1α retards neurodegeneration in Sandhoff disease mice. Proc Natl Acad Sci USA. 2004. 101:8425–8430.
Article11. Tybulewicz VL, Tremblay ML, LaMarca ME, Willemsen P, Stubblefield BK, Winfield S, Zablocka B, Sidransky E, Martin BM, Huang SP, Mintzer KN, Westphal H, Mulligan RO, Ginns EI. Animal model of Gaucher's disease from targeted disruption of the mouse glucocerebrosidase gene. Nature. 1992. 357:407–410.
Article12. Min KJ, Yang MS, Jou I, Joe EH. Protein kinase A mediates microglial activation induced by plasminogen and gangliosides. Exp Mol Med. 2004. 36:461–467.
Article13. Pelled D, Shogomori H, Futerman AH. The increased sensitivity of neurons with elevated glucocerebroside to neurotoxic agents can be reversed by imiglucerase. J Inherit Metab Dis. 2000. 23:175–184.
Article14. Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J Immunol. 1988. 141:2407–2412.15. Korkotian E, Schwarz A, Pelled D, Schwarzmann G, Segal M, Futerman AH. Elevation of intracellular glucosylceramide levels results in an increase in endoplasmic reticulum density and in functional calcium stores in cultured neurons. J Biol Chem. 1999. 274:21673–21678.
Article16. Orvisky E, Park JK, LaMarca ME, Ginns EI, Martin BM, Tayebi N, Sidransky E. Glucosylsphingosine accumulation in tissues from patients with Gaucher disease: Correlation with phenotype and genotype. Mol Genet Metab. 2002. 76:262–270.
Article17. Orvisky E, Sidransky E, McKinney CE, LaMarca ME, Samimi R, Krasnewich D, Martin BM, Ginns EI. Glucosylsphingosine accumulation in mice and patients with type 2 Gaucher disease begins early in gestation. Pediatr Res. 2000. 48:233–237.
Article18. Lloyd-Evans E, Pelled D, Riebeling G, Futerman AH. Lyso-glycosphingolipids mobilize calcium from brain microsomes via multiple mechanisms. Biochem J. 2003. 375:561–565.
Article19. Schueler UH, Kolter T, Kaneski CR, Blusztajn JK, Herkenham M, Sandhoff K, Brady RO. Toxicity of glucosylsphingosine to cultured neuronal cells: a model system for assessing neuronal damage in Gaucher disease type 2 and 3. Neurobiol Dis. 2003. 14:595–601.20. Raas-Rothschild A, Pankova-Kholmyansky I, Kacher Y, Futerman AH. Glycosphingolipidoses: beyond the enzymatic defect. Glycoconj J. 2004. 21:295–304.
Article21. Shoenfeld Y, Gallant LA, Shaklai M, Livni E, Djaaldetti M, Pinkhas J. Gaucher's disease: a disease with chronic stimulation of the immune system. Arch Pathol Lab Med. 1982. 106:388–391.22. Kluth DC, Rees AJ. Inhibiting inflammatory cytokines. Semin Nephrol. 1996. 16:576–582.23. Liu B, Hong JS. Role of microglia in inflammation mediated neurodegenerative disease: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003. 304:1–7.24. McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1998. 38:1285–1291.
Article25. Raine CS. Multiple sclerosis: immune system molecule expression in the central nervous system. J Neuropathol Exp Neurol. 1994. 53:328–337.
Article26. Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmalcol. 2003. 54:469–487.27. Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001. 2:907–916.
Article28. Faber JL. Mechanisms of cell injury by activated oxygen species. Environ Health Perspect. 1994. 102:17–24.29. Wang T, Qin L, Liu B, Liu Y, Wilson B, Eling TE, Langenbach R, Taniura S, Hong JS. Role of reactive oxygen species in LPS-induced production of prostaglandin E2 in microglia. J Neurochem. 2004. 88:939–947.
Article30. Lal MA, Brismar H, Eklof AC, Aperia A. Role of oxidative stress in advanced glycation end product-induced mesangial cell activation. Kidney Int. 2002. 61:2006–2014.
Article31. Rosenberger J, Petrovics G, Buzas B. Oxidative stress induces proorphanin FQ and proenkephalin gene expression in astrocytes through p38- and ERK-MAP kinases and NF-kappaB. J Nerurochem. 2001. 79:35–44.32. Myerowitz R, Lawson D, Mizukami H, Mi Y, Tifft CJ, Proia RL. Molecular pathophysiology in Tay-Sachs and Sandhoff diseases as revealed by gene expression profiling. Hum Mol Genet. 2002. 11:1343–1350.
Article33. Wada R, Tifft CJ, Proia RL. Microglial activation precedes acute neurodegeneration in Sandhoff disease and is suppressed by bone marrow transplantation. Proc Natl Acad Sci USA. 2000. 97:10954–10959.
Article34. Liu Y, Suzuki K, Reed JD, Grinberg A, Westphal H, Hoffmann A, Döring T, Sandhoff K, Proia RL. Mice with type 2 and 3 Gaucher disease point mutations generated by a single insertion mutagenesis procedure (SIMP). Proc Natl Acad Sci USA. 1998. 95:2503–2508.
Article35. Xu YH, Quinn B, Witte D, Grabowski GA. Viable mouse models of acid beta-glucosidase deficiency: the defect in Gaucher disease. Am J Pathol. 2003. 163:2093–2101.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Downregulation of neurotrophic factors in the brain of a mouse model of Gaucher disease: implications for neuronal loss in Gaucher disease
- Modulation of the action of proinflammatory cytokines on neutrophil function by pentoxifylline
- Gaucher's Disease: A Report of Two Cases in Homozygous Twins
- Two cases of Gaucher's disease in brothers
- Two cases of Gaucher disease in brother and sister