J Korean Med Sci.
2004 Aug;19(4):560-566. 10.3346/jkms.2004.19.4.560.
Norcantharidin Induces Human Melanoma A375-S2 Cell Apoptosis through Mitochondrial and Caspase Pathways
- Affiliations
-
- 1China-Japan Research Institute of Medical and Pharmaceutical Sciences, Shenyang Pharmaceutical University, Shenyang, China. ikejimat@vip.sina.com
- 2Department of Pharmacology, Shenyang Pharmaceutical University, Shenyang, China.
- 3Department of Clinical and Biomedical Sciences, Showa Pharmaceutical University, Tokyo, Japan.
- KMID: 2157686
- DOI: http://doi.org/10.3346/jkms.2004.19.4.560
Abstract
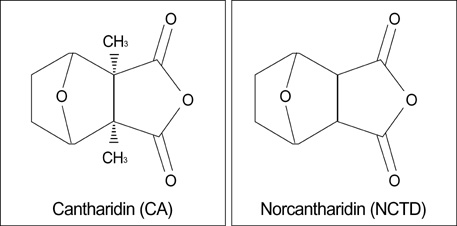
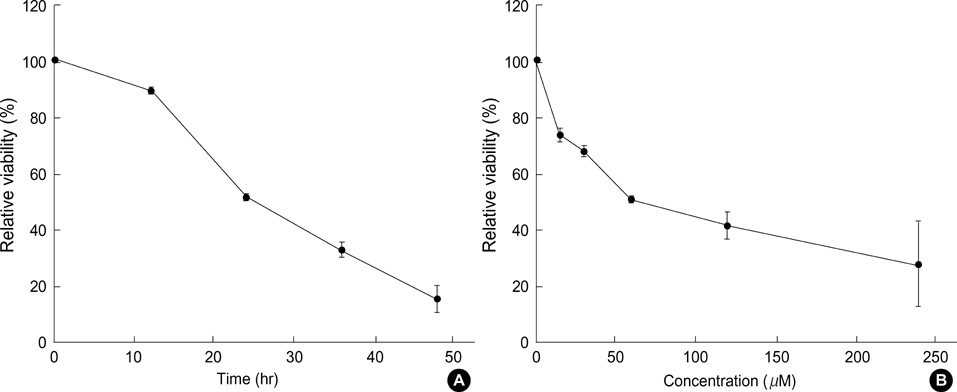
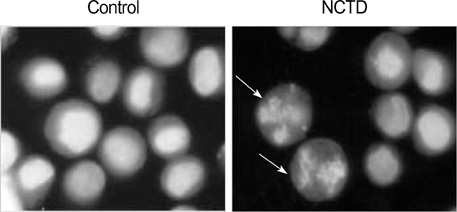
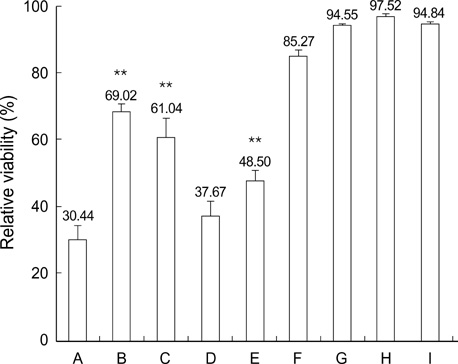
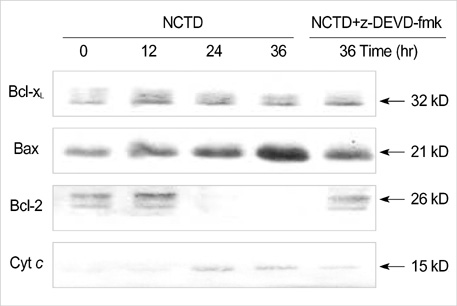
- Norcantharidin (NCTD) is the demethylated form of cantharidin, which is the active substance of mylabris. To examine the pathway of NCTD-induced A375-S2 cell death, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-dipheyltetrazolium bromide (MTT) assay, photomicroscopical observation, DNA agarose gel electrophoresis, caspase activity assay and Western blot analysis were carried out. A375-S2 cells treated with NCTD exhibited several typical characteristics of apoptosis. The inhibitory effect of NCTD on human melanoma, A375-S2 cells, was partially reversed by the inhibitors of pan-caspase, caspase-3 and caspase-9. The activities of caspase-3 and -9 were significantly increased after treatment with NCTD at different time. The expression of inhibitor of caspase-activated DNase was decreased in a time-dependent manner, simultaneously, the ratio of Bcl-2/Bax or Bcl-xL/Bax was decreased and the expression ratio of proteins could be reversed by caspase-3 inhibitor. The expression of cytochrome c in cytosol was increased after NCTD treatment and caspase- 3 inhibitor had no significant effect on the up-regulation of cytochrom c. These results suggest that NCTD induced A375-S2 cell apoptosis and the activation of caspase and mitochondrial pathway were involved in the process of NCTD-induced A375-S2 cell apoptosis.
Keyword
MeSH Terms
-
Animals
Apoptosis/*physiology
Bicyclo Compounds, Heterocyclic/chemistry/metabolism/*pharmacology
Caspases/antagonists & inhibitors/*metabolism
Cell Line, Tumor/*drug effects
Cell Shape
DNA Fragmentation
Enzyme Activation
Humans
Mitochondria/*metabolism
Molecular Structure
Proto-Oncogene Proteins c-bcl-2/metabolism
Signal Transduction/*physiology
Figure
Reference
-
1. Peng F, Wei YQ, Tian L, Yang L, Zhao X, Lu Y, Mao YQ, Kan B, Lei S, Wang GS, Jiang Y, Wang QR, Luo F, Zou LQ, Liu JY. Induction of apoptosis by norcantharidin in human colorectal carcinoma cell lines: involvement of the CD95 receptor/ligand. J Cancer Res Clin Oncol. 2002. 128:223–230.
Article2. Hong CY, Huang SC, Lin SK, Lee JJ, Chueh LL, Lee CH, Lin JH, Hsiao M. Norcantharidin-induced post-G2/M apoptosis is dependent on wild-type p53 gene. Biochem Biophys Res Commun. 2000. 276:278–285.
Article3. Rong Y, Liang FY, Chen L, Du HJ, Liu LY, Sun HL, An W. Norcantharidin induces apoptosis in human breast cancer cell line. Cancer. 2000. 19:1077–1081.4. Kim SO, Han J. Pan-caspase inhibitor zVAD enhances cell death in RAW 246.7 macrophages. J Endotoxin Res. 2001. 7:292–296.5. Kawazoe N, Watabe M, Masuda Y, Nakajo S, Nakaya K. Tiami1 is involved in the regulation of bufalin-induced apoptosis in human leukemia cells. Oncogene. 1999. 18:2413–2421.6. Hill PA, Tumber A, Meikle MC. Multiple extracellular signals promote osteoblast survival and apoptosis. Endocrinology. 1997. 138:3849–3858.7. Mizukami S, Kikuchi K, Higuchi T, Urano Y, Mashima T, Tsuruo T, Nagano T. Imaging of caspase-3 activation in HeLa cells stimulated with etoposide using a novel fluorescent probe. FEBS Lett. 1999. 453:356–360.
Article8. Zhang Y, Fujita N, Tsuruo T. Caspase-mediated cleavage of p21Waf1/Cip1 converts cells from growth arrest to undergoing apoptosis. Oncogene. 1999. 18:1131–1138.9. Kirsch DG, Doseff A, Chau BN, Lim DS, de Souza-Pinto NC, Hansford R, Kastan MB, Lazebnik YA, Hardwick JM. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J Biol Chem. 1999. 274:21155–21161.
Article10. Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis and its inhibitor ICAD. Nature. 1998. 391:43–50.
Article11. Doerfler P, Forbush KA, Perlmutter RM. Caspase enzyme activity is not essential for apoptosis during thymocyte development. J Immunol. 2000. 164:4071–4079.
Article12. Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997. 278:1966–1968.
Article13. Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998. 281:1309–1312.14. Nagata S. Apoptosis by death factor. Cell. 1997. 88:355–365.
Article15. Wang NS, Unkila MT, Reineks EZ, Distelhorst CW. Transient expression of wild-type or mitochondrially targeted Bcl-2 induces apoptosis, whereas transient expression of endoplasmic reticulum-targeted Bcl-2 is protective against Bax-induced cell death. J Biol Chem. 2001. 276:44117–44128.
Article16. Klefstrom J, Verschuren EW, Evan G. c-Myc augments the apoptotic activity of cytosolic death receptor signaling proteins by engaging the mitochondrial apoptotic pathway. J Biol Chem. 2002. 277:43224–43232.
Article17. Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001. 412:95–99.
Article18. Gamet-Payraste L, Lumeau S, Cassar G, Dupont MA, Chevolleau S, Gasc N, Tulliez J, Terce F. Sulforaphane, naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000. 60:1426–1433.19. Sarin A, Haddad EK, Henkart PA. Caspase dependence of target cell damage induced by cytotoxic T lymphocytes. J Immunol. 1998. 161:2810–2816.20. Charrier L, Jarry A, Toquet C, Bou-Hanna C, Chedorge M, Denis M, Vallette G, Laboisse CL. Growth phase-dependent expression of ICAD-L/DFF45 modulates the pattern of apoptosis in human colonic cancer cells. Cancer Res. 2002. 62:2169–2174.21. Kim YM, Talanian RV, Billiar TR. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanism. J Biol Chem. 1997. 272:31138–31148.22. Herrmann M, Lorenz HM, Voll R, Grunke M, Woith W, Kalden JR. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res. 1994. 22:5506–5507.
Article23. Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. 1989. 2nd ed. Cold Spring Harbor Laboratory Press;880–898.24. Villa P, Kaufmann SH, Earnshaw WC. Caspase and caspase inhibitors. Trends in Biochem Sci. 1997. 22:388–393.25. Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998. 391:96–99.
Article26. Yin XM, Oltvai ZN, Korsmeyer SJ. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994. 369:321–323.
Article27. Cheng EH, Levine B, Boise LH, Thompson CB, Hardwick JM. Bax-independent inhibition of apoptosis by Bcl-xL. Nature. 1996. 379:554–556.
Article28. Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998. 281:1322–1326.
Article29. Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997. 387:773–776.
Article30. Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997. 275:1129–1132.
Article31. Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997. 275:1132–1136.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- IL-1beta Acts in Synergy with Endogenous IL-1beta in A375-S2 Human Melanoma Cell Apoptosis Through Mitochondrial Pathway
- Control of Mitochondrial Dynamics by Fas-induced Caspase-8 Activation in Hippocampal Neurons
- Apoptotic Effects of Co-Treatment with a Chios Gum Mastic and Eugenol on G361 Human Melanoma Cells
- Caspases Activation in Ultraviolet B-induced Apoptosis of G361 Human Melanoma Cell Line
- Effect of apoptosis on G361 cells by Cimicifuga rhizoma extract