J Pathol Transl Med.
2015 Jul;49(4):318-324. 10.4132/jptm.2015.06.01.
Cancers with Higher Density of Tumor-Associated Macrophages Were Associated with Poor Survival Rates
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. yjparkmd@snu.ac.kr
- 2Department of Internal Medicine, Eulji University School of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, National Medical Center, Seoul, Korea.
- 4Department of Pathology, SMG-SNU Boramae Medical Center, Seoul, Korea.
- 5Lee Gil Ya Cancer and Diabetes Institute, Gachon University Graduate School of Medicine, Incheon, Korea.
- KMID: 2151141
- DOI: http://doi.org/10.4132/jptm.2015.06.01
Abstract
- BACKGROUND
Macrophages are a component of a tumor's microenvironment and have various roles in tumor progression and metastasis. This study evaluated the relationships between tumor-associated macrophage (TAM) density and clinical outcomes in 14 different types of human cancers.
METHODS
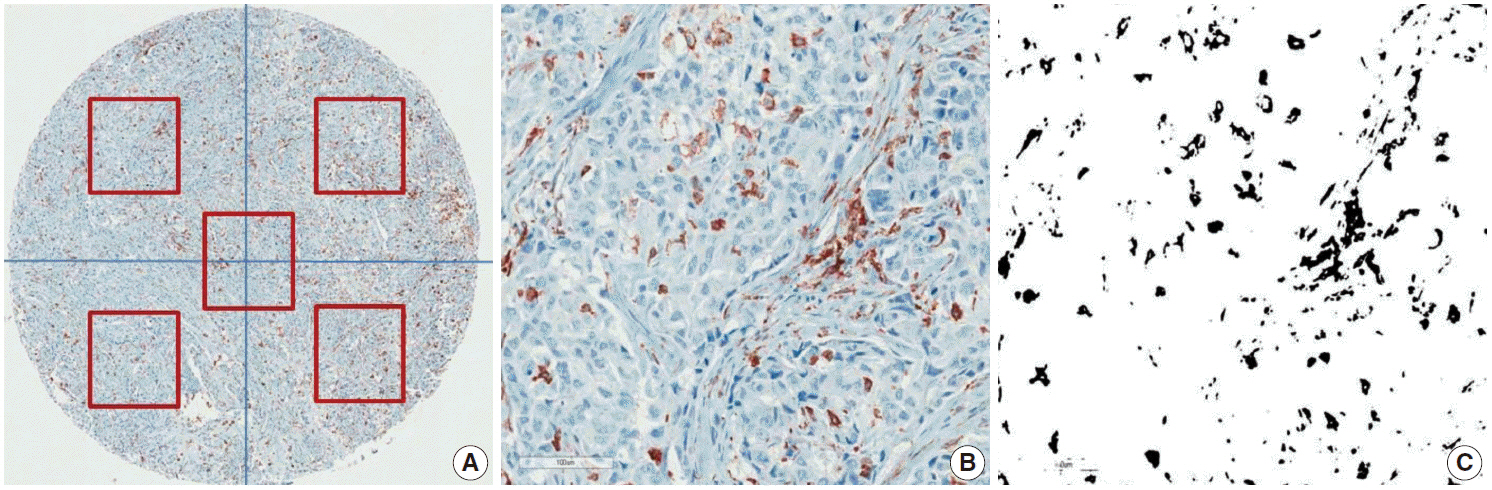
We investigated TAM density in human tissue microarray sections from 14 different types of human cancers (n = 266) and normal thyroid, lung, and breast tissues (n = 22). The five-year survival rates of each cancer were obtained from the 2011 Korea Central Cancer Registry.
RESULTS
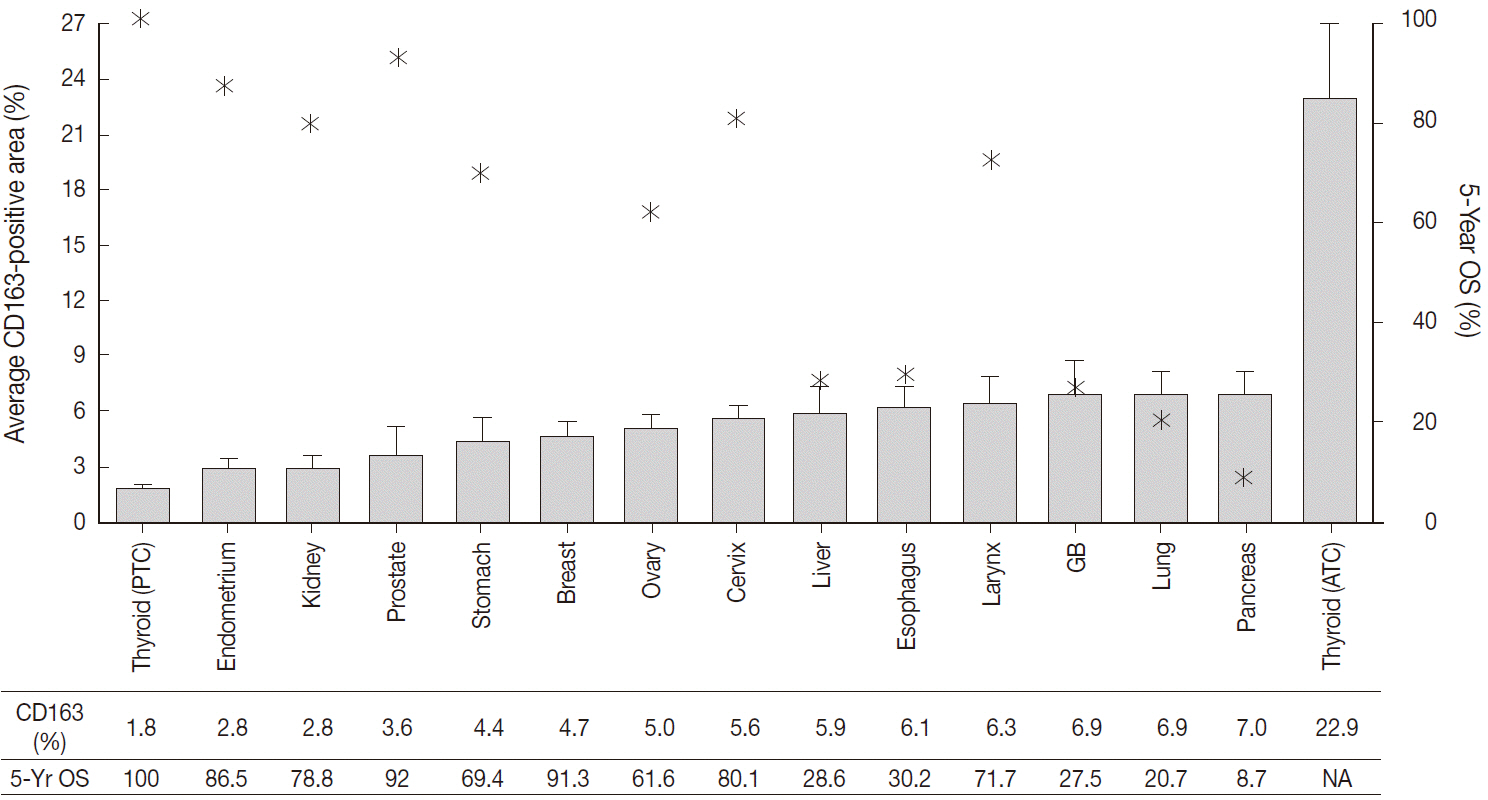
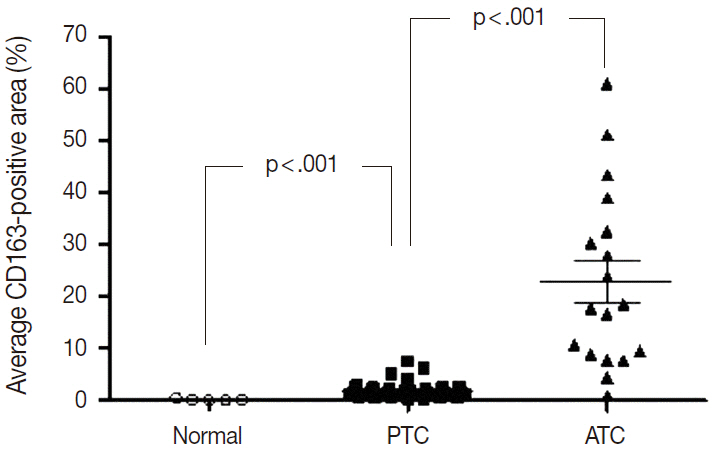
Among 13 human cancers, excluding thyroid cancer, pancreas, lung, and gallbladder cancers had the highest density of CD163-positive macrophages (7.0+/-3.5%, 6.9+/-7.4%, and 6.9 +/- 5.5%, respectively). The five-year relative survival rates of these cancers (pancreas, 8.7%; lung, 20.7%; gallbladder, 27.5%) were lower than those of other cancers. The histological subtypes in thyroid cancer exhibited significantly different CD163-positive macrophages densities (papillary, 1.8 +/- 1.6% vs anaplastic, 22.9 +/- 17.1%; p < .001), but no significant difference between histological subtypes was detected in lung and breast cancers. Moreover, there was no significant difference in CD163-positive macrophages densities among the TNM stages in lung, breast, and thyroid cancers.
CONCLUSIONS
Cancers with higher TAM densities (pancreas, lung, anaplastic thyroid, and gallbladder) were associated with poor survival rate.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
The Expression and Relationship of CD68-Tumor-Associated Macrophages and Microvascular Density With the Prognosis of Patients With Laryngeal Squamous Cell Carcinoma
Shujun Sun, Xinliang Pan, Limin Zhao, Jianming Zhou, Hongzeng Wang, Yonghong Sun
Clin Exp Otorhinolaryngol. 2016;9(3):270-277. doi: 10.21053/ceo.2015.01305.Clinicopathological Features and Molecular Signatures of Lateral Neck Lymph Node Metastasis in Papillary Thyroid Microcarcinoma
Jinsun Lim, Han Sai Lee, Jin-Hyung Heo, Young Shin Song
Endocrinol Metab. 2024;39(2):324-333. doi: 10.3803/EnM.2023.1885.
Reference
-
1. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002; 420:860–7.
Article2. Cho SW. Interactions between immune cells and tumor cells. J Korean Thyroid Assoc. 2013; 6:96–100.
Article3. Zhang QW, Liu L, Gong CY, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012; 7:e50946.
Article4. Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002; 196:254–65.
Article5. Kim S, Cho SW, Min HS, et al. The expression of tumor-associated macrophages in papillary thyroid carcinoma. Endocrinol Metab (Seoul). 2013; 28:192–8.
Article6. Zeni E, Mazzetti L, Miotto D, et al. Macrophage expression of interleukin-10 is a prognostic factor in nonsmall cell lung cancer. Eur Respir J. 2007; 30:627–32.
Article7. Campbell MJ, Tonlaar NY, Garwood ER, et al. Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Cancer Res Treat. 2011; 128:703–11.
Article8. Sato S, Hanibuchi M, Kuramoto T, et al. Macrophage stimulating protein promotes liver metastases of small cell lung cancer cells by affecting the organ microenvironment. Clin Exp Metastasis. 2013; 30:333–44.
Article9. Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007; 13:1472–9.
Article10. Ohno S, Inagawa H, Dhar DK, et al. The degree of macrophage infiltration into the cancer cell nest is a significant predictor of survival in gastric cancer patients. Anticancer Res. 2003; 23:5015–22.11. Welsh TJ, Green RH, Richardson D, Waller DA, O’Byrne KJ, Bradding P. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol. 2005; 23:8959–67.
Article12. Kawai O, Ishii G, Kubota K, et al. Predominant infiltration of macrophages and CD8(+) T cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer. 2008; 113:1387–95.13. Ohno S, Ohno Y, Suzuki N, et al. Correlation of histological localization of tumor-associated macrophages with clinicopathological features in endometrial cancer. Anticancer Res. 2004; 24:3335–42.14. Cho SW, Kim YA, Sun HJ, et al. Therapeutic potential of Dickkopf-1 in wild-type BRAF papillary thyroid cancer via regulation of betacatenin/E-cadherin signaling. J Clin Endocrinol Metab. 2014; 99:E1641–9.15. Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2011. Cancer Res Treat. 2014; 46:109–23.
Article16. Cornett WR, Sharma AK, Day TA, et al. Anaplastic thyroid carcinoma: an overview. Curr Oncol Rep. 2007; 9:152–8.
Article17. Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol. 2006; 13:453–64.
Article18. Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol). 2010; 22:486–97.
Article19. Haymart MR, Banerjee M, Yin H, Worden F, Griggs JJ. Marginal treatment benefit in anaplastic thyroid cancer. Cancer. 2013; 119:3133–9.
Article20. Lin JD, Chen ST, Hsueh C, Chao TC. A 29-year retrospective review of papillary thyroid cancer in one institution. Thyroid. 2007; 17:535–41.
Article21. Murri AM, Hilmy M, Bell J, et al. The relationship between the systemic inflammatory response, tumour proliferative activity, T-lymphocytic and macrophage infiltration, microvessel density and survival in patients with primary operable breast cancer. Br J Cancer. 2008; 99:1013–9.
Article22. Medrek C, Pontén F, Jirström K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012; 12:306.
Article23. Ohtaki Y, Ishii G, Nagai K, et al. Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. J Thorac Oncol. 2010; 5:1507–15.
Article24. Zhang BC, Gao J, Wang J, Rao ZG, Wang BC, Gao JF. Tumor-associated macrophages infiltration is associated with peritumoral lymphangiogenesis and poor prognosis in lung adenocarcinoma. Med Oncol. 2011; 28:1447–52.
Article25. Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010; 10:112.
Article26. Al-Shibli K, Al-Saad S, Donnem T, Persson M, Bremnes RM, Busund LT. The prognostic value of intraepithelial and stromal innate immune system cells in non-small cell lung carcinoma. Histopathology. 2009; 55:301–12.
Article27. Steele RJ, Eremin O, Brown M, Hawkins RA. Oestrogen receptor concentration and macrophage infiltration in human breast cancer. Eur J Surg Oncol. 1986; 12:273–6.28. Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004; 4:71–8.
Article29. Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011; 9:216.
Article30. Ambarus CA, Krausz S, van Eijk M, et al. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods. 2012; 375:196–206.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The role of tumor-associated macrophages on microvessel density after neoadjuvant chemotherapy in tongue cancer
- The Expression and Relationship of CD68-Tumor-Associated Macrophages and Microvascular Density With the Prognosis of Patients With Laryngeal Squamous Cell Carcinoma
- Prognostic Analysis in Colorectal Cancers Requiring Emergency Operations
- The Expression of Tumor-Associated Macrophages in Papillary Thyroid Carcinoma
- Tumor-Associated Macrophages as Potential Prognostic Biomarkers of Invasive Breast Cancer