Immune Netw.
2013 Aug;13(4):133-140. 10.4110/in.2013.13.4.133.
Mesenchymal Stem Cell Lines Isolated by Different Isolation Methods Show Variations in the Regulation of Graft-versus-host Disease
- Affiliations
-
- 1Translational Research Center, Inha University School of Medicine, Incheon 400-711, Korea. msjeon@inha.ac.kr, sunuksong@inha.ac.kr
- 2Department of Radiation Oncology, Inha University School of Medicine, Incheon 400-711, Korea.
- 3Inha Research Institute for Medical Sciences, Inha University School of Medicine, Incheon 400-711, Korea.
- 4Department of Drug Development, Inha University School of Medicine, Incheon 400-711, Korea.
- 5HomeoTherapy Co. Ltd., Incheon 400-711, Korea.
- KMID: 2150778
- DOI: http://doi.org/10.4110/in.2013.13.4.133
Abstract
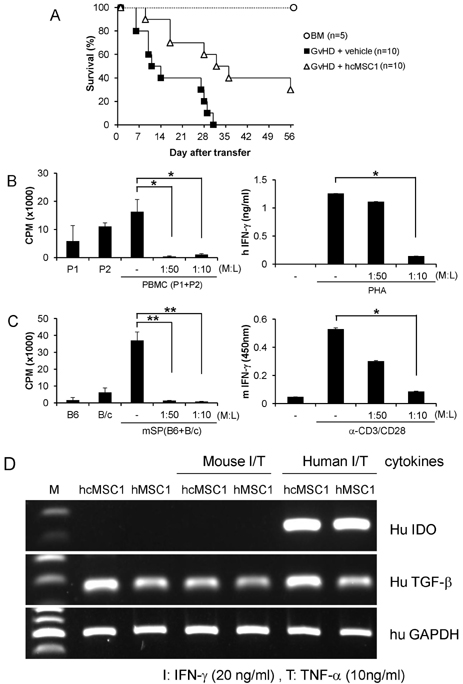
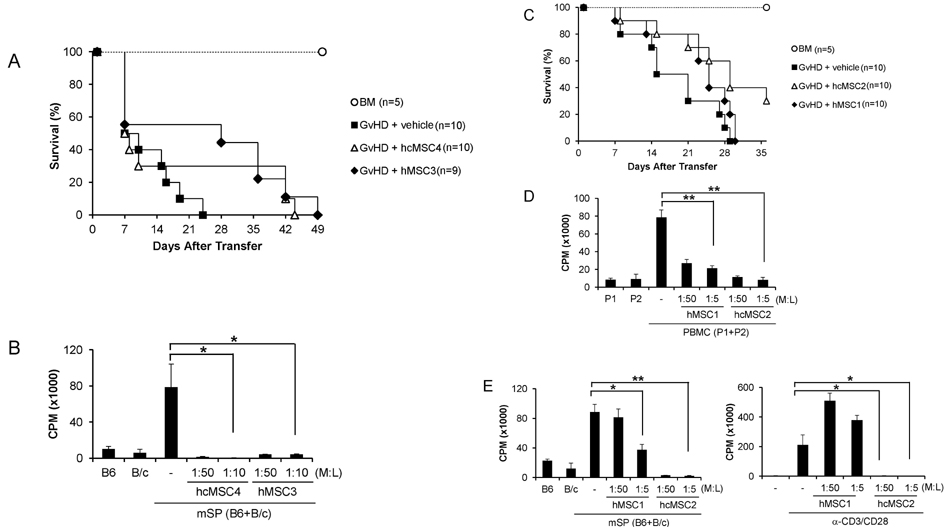
- Since the discovery of the immunomodulation property of mesenchymal stem cells (MSCs) about a decade ago, it has been extensively investigated whether MSCs can be used for the treatment of immune-related diseases, such as graft-versus-host disease (GvHD). However, how to evaluate the efficacy of human MSCs for the clinical trial is still unclear. We used an MHC-mismatched model of GvHD (B6 into BALB/c). Surprisingly, the administration of the human MSCs (hMSCs) could reduce the GvHD-related mortality of the mouse recipients and xenogeneically inhibit mouse T-cell proliferation and IFN-gamma production in vitro. We recently established a new protocol for the isolation of a homogeneous population of MSCs called subfractionation culturing methods (SCM), and established a library of clonal MSC lines. Therefore, we also investigated whether MSCs isolated by the conventional gradient centrifugation method (GCM) and SCM show different efficacy in vivo. Intriguingly, clonal hMSCs (hcMSCs) isolated by SCM showed better efficacy than hMSCs isolated by GCM. Based on these results, the MHC-mismatched model of GvHD may be useful for evaluating the efficacy of human MSCs before the clinical trial. The results of this study suggest that different MSC lines may show different efficacy in vivo and in vitro.
MeSH Terms
Figure
Cited by 1 articles
-
Molecular Characterization of Neurally Differentiated Human Bone Marrow-derived Clonal Mesenchymal Stem Cells
TacGhee Yi, Hyun-Joo Lee, Yun-Kyoung Cho, Myung-Shin Jeon, Sun U. Song
Immune Netw. 2014;14(1):54-65. doi: 10.4110/in.2014.14.1.54.
Reference
-
1. Goker H, Haznedaroglu IC, Chao NJ. Acute graft-vs-host disease: pathobiology and management. Exp Hematol. 2001; 29:259–277.
Article2. Menillo SA, Goldberg SL, McKiernan P, Pecora AL. Intraoral psoralen ultraviolet A irradiation (PUVA) treatment of refractory oral chronic graft-versus-host disease following allogeneic stem cell transplantation. Bone Marrow Transplant. 2001; 28:807–808.
Article3. Deeg HJ. How I treat refractory acute GVHD. Blood. 2007; 109:4119–4126.
Article4. Rager A, Frey N, Goldstein SC, Reshef R, Hexner EO, Loren A, Luger SM, Perl A, Tsai D, Davis J, Vozniak M, Smith J, Stadtmauer EA, Porter DL. Inflammatory cytokine inhibition with combination daclizumab and infliximab for steroid-refractory acute GVHD. Bone Marrow Transplant. 2011; 46:430–435.
Article5. Schub N, Günther A, Schrauder A, Claviez A, Ehlert C, Gramatzki M, Repp R. Therapy of steroid-refractory acute GVHD with CD52 antibody alemtuzumab is effective. Bone Marrow Transplant. 2011; 46:143–147.
Article6. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997; 276:71–74.
Article7. Branch MJ, Hashmani K, Dhillon P, Jones DR, Dua HS, Hopkinson A. Mesenchymal stem cells in the human corneal limbal stroma. Invest Ophthalmol Vis Sci. 2012; 53:5109–5116.
Article8. Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003; 75:389–397.
Article9. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002; 99:3838–3843.
Article10. Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005; 105:4120–4126.
Article11. Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006; 24:74–85.
Article12. Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006; 107:367–372.
Article13. Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005; 105:2821–2827.
Article14. Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004; 363:1439–1441.
Article15. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O. Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008; 371:1579–1586.
Article16. Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, Omazic B, Aschan J, Barkholt L, Le Blanc K. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006; 81:1390–1397.
Article17. Allison M. Genzyme backs osiris, despite prochymal flop. Nat Biotechnol. 2009; 27:966–967.
Article18. Li WJ, Chiang H, Kuo TF, Lee HS, Jiang CC, Tuan RS. Evaluation of articular cartilage repair using biodegradable nanofibrous scaffolds in a swine model: a pilot study. J Tissue Eng Regen Med. 2009; 3:1–10.
Article19. Liao W, Xie J, Zhong J, Liu Y, Du L, Zhou B, Xu J, Liu P, Yang S, Wang J, Han Z, Han ZC. Therapeutic effect of human umbilical cord multipotent mesenchymal stromal cells in a rat model of stroke. Transplantation. 2009; 87:350–359.
Article20. Yan H, Yu C. Repair of full-thickness cartilage defects with cells of different origin in a rabbit model. Arthroscopy. 2007; 23:178–187.
Article21. Bruck F, Belle L, Lechanteur C, de Leval L, Hannon M, Dubois S, Castermans E, Humblet-Baron S, Rahmouni S, Beguin Y, Briquet A, Baron F. Impact of bone marrow-derived mesenchymal stromal cells on experimental xenogeneic graft-versus-host disease. Cytotherapy. 2013; 15:267–279.
Article22. Song SU, Kim CS, Yoon SP, Kim SK, Lee MH, Kang JS, Choi GS, Moon SH, Choi MS, Cho YK, Son BK. Variations of clonal marrow stem cell lines established from human bone marrow in surface epitopes, differentiation potential, gene expression, and cytokine secretion. Stem Cells Dev. 2008; 17:451–461.
Article23. Jeon MS, Yi TG, Lim HJ, Moon SH, Lee MH, Kang JS, Kim CS, Lee DH, Song SU. Characterization of mouse clonal mesenchymal stem cell lines established by subfractionation culturing method. World J Stem Cells. 2011; 3:70–82.
Article24. Saito T, Kuang JQ, Bittira B, Al-Khaldi A, Chiu RC. Xenotransplant cardiac chimera: immune tolerance of adult stem cells. Ann Thorac Surg. 2002; 74:19–24.
Article25. Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000; 6:1282–1286.
Article26. Jeon MS, Lim HJ, Yi TG, Im MW, Yoo HS, Choi JH, Choi EY, Song SU. Xenoreactivity of human clonal mesenchymal stem cells in a major histocompatibility complex-matched allogeneic graft-versus-host disease mouse model. Cell Immunol. 2010; 261:57–63.
Article27. Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008; 2:141–150.
Article28. Dazzi F, Marelli-Berg FM. Mesenchymal stem cells for graft-versus-host disease: close encounters with T cells. Eur J Immunol. 2008; 38:1479–1482.
Article29. Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007; 109:228–234.
Article30. Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008; 222:206–221.
Article31. Baron F, Storb R. Mesenchymal stromal cells: a new tool against graft-versus-host disease? Biol Blood Marrow Transplant. 2012; 18:822–840.
Article32. Sudres M, Norol F, Trenado A, Gregoire S, Charlotte F, Levacher B, Lataillade JJ, Bourin P, Holy X, Vernant JP, Klatzmann D, Cohen JL. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol. 2006; 176:7761–7767.
Article33. Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, Genrich K, Mehrotra S, Setty S, Smith B, Bartholomew A. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008; 38:1745–1755.
Article34. Yanez R, Lamana ML, Garcia-Castro J, Colmenero I, Ramirez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006; 24:2582–2591.
Article35. Jang MJ, Kim HS, Lee HG, Kim GJ, Jeon HG, Shin HS, Chang SK, Hur GH, Chong SY, Oh D, Chung HM. Placenta-derived mesenchymal stem cells have an immunomodulatory effect that can control acute graft-versus-host disease in mice. Acta Haematol. 2013; 129:197–206.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Mesenchymal Stem Cell Lines Isolated by Different Isolation Methods Show Variations in the Regulation of Graft-versus-host Disease
- The potential use of mesenchymal stem cells in hematopoietic stem cell transplantation
- A case of pneumomediastinum combined with chronic graft-versus-host disease following allogeneic hematopoietic stem cell transplantation
- Acute Cutaneous Graft-Versus-Host Reaction
- Clinical applications of mesenchymal stem cells