Immune Netw.
2010 Apr;10(2):64-74. 10.4110/in.2010.10.2.64.
Polybrominated Diphenyl Ethers Orally Administration to Mice Were Tansferred to Offspring during Gestation and Lactation with Disruptions on the Immune System
- Affiliations
-
- 1Immnotoxicity Division, National Institute of Toxicological Research, Korea Food and Drug Administration, Seoul, Korea. parkkl@kfda.go.kr
- KMID: 2150661
- DOI: http://doi.org/10.4110/in.2010.10.2.64
Abstract
-
BACKGROUND: The present study was undertaken to examine the immunological effects of pentabrominated diphenyl ether (penta-BDE) and decabrominated diphenyl ether (deca-BDE) on the immune system of the dams and the developmental immune system of the offsprings.
METHODS
In this study, mated female C57BL/6J mice were orally administered penta-BDE, deca-BDE or corn oil for 5 weeks, from gestational day 6 to lactational day 21.
RESULTS
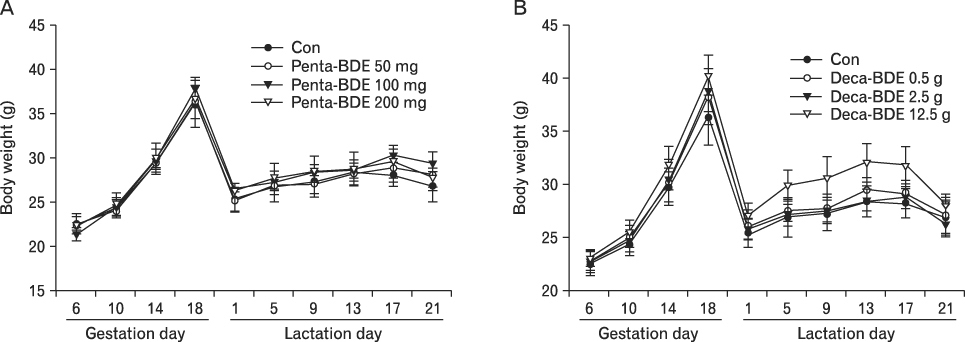
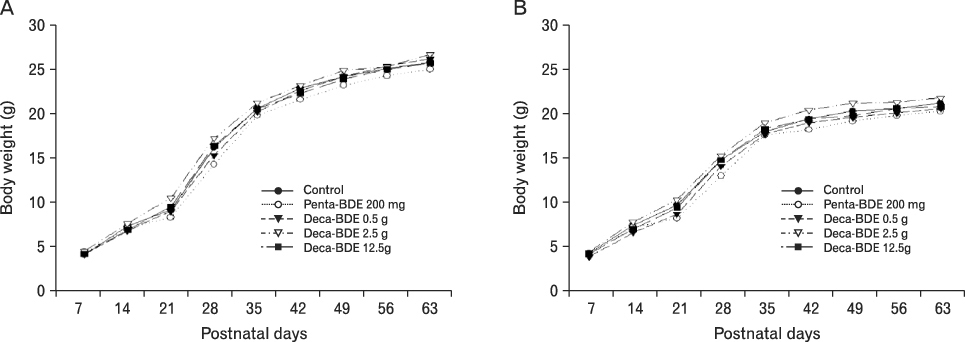
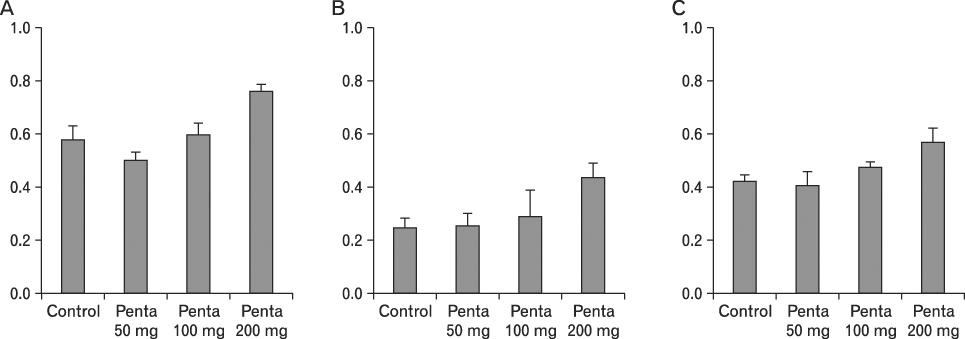
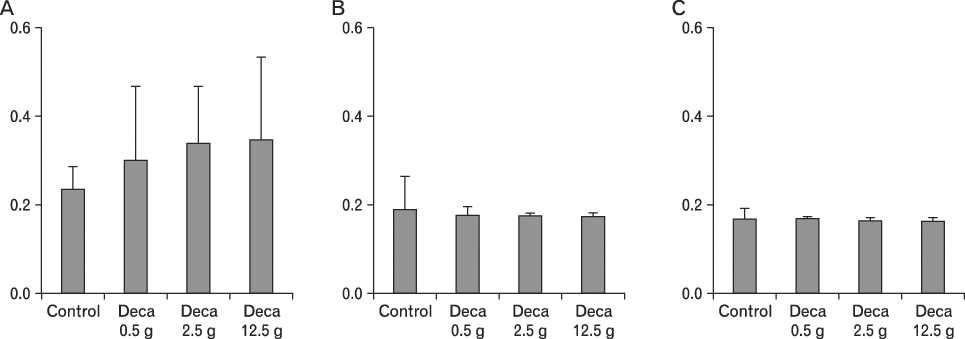
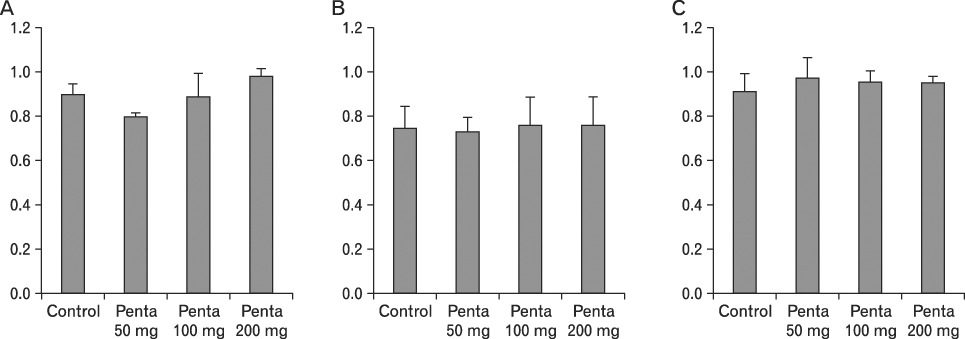
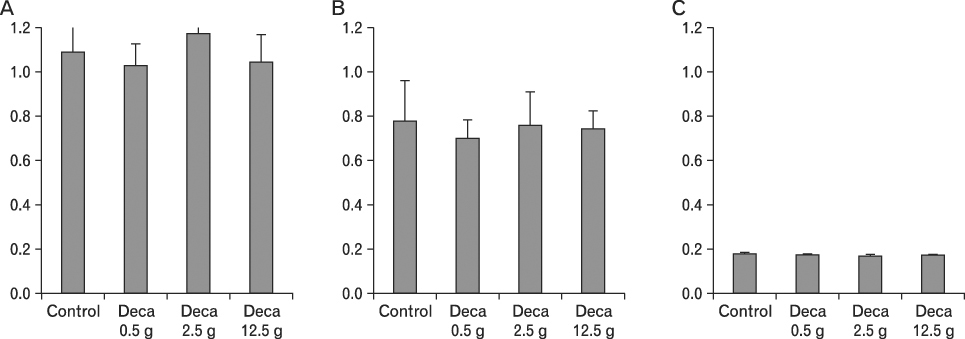
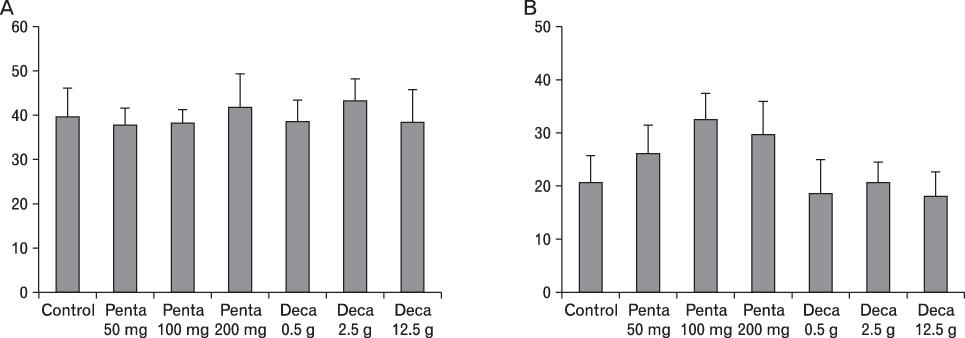
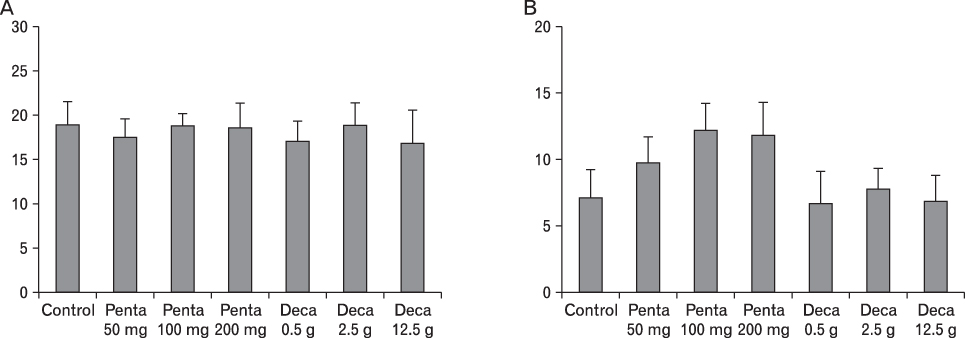
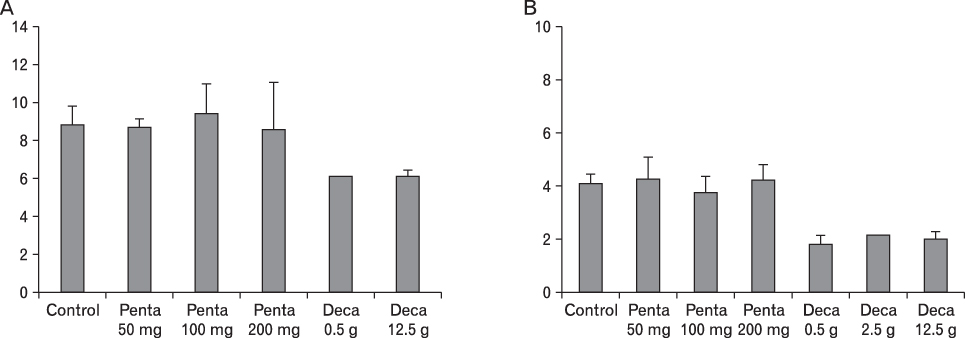
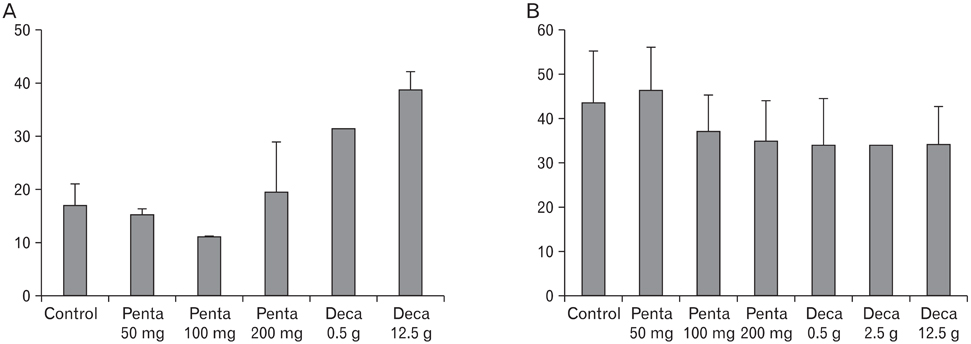
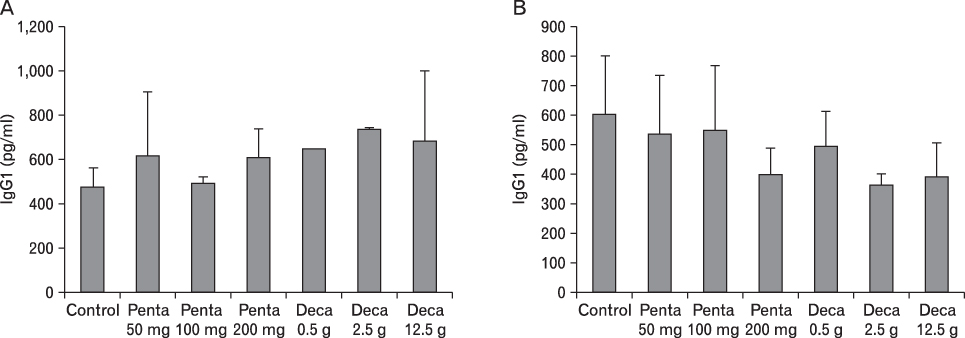
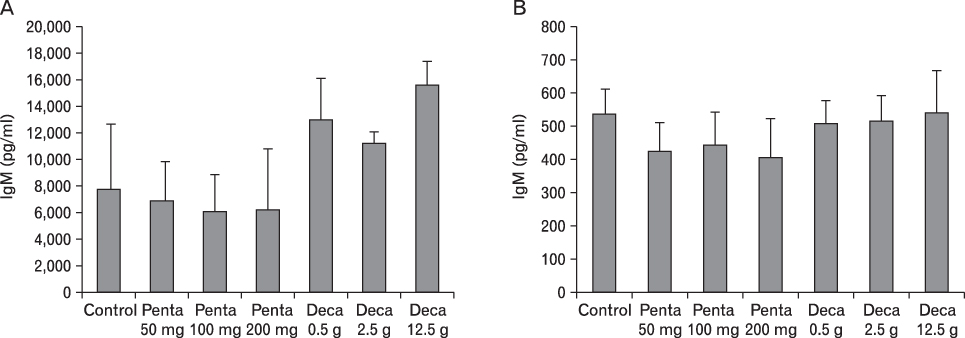
The body weight of PND21 exposed to penta-BDE was significantly decreased relative to control mice, but that of post-natal day 63 (PND63) were recovered. Orally dosed dams with penta-BDE had significantly smaller absolute and relative spleen masses than control mice. Absolute and relative spleen and thymus masses of PND21 exposed to penta-BDE were significantly decreased over control. The exposure of dams and PND21 with penta-BDE reduced the number of splenocytes and thymocytes. As results of hematologic analysis, percentage WBC and percentage neutrophils increased in dams with deca-BDE. Splenic T cell proliferation in dams and PND21 exposed to penta-BDE was increased, and there were no significant difference in splenic B cell proliferation in all treatment groups. As results of flow cytometric analysis of splenocyte, percentage total T cell, Th cell and Tc cell in PND21 exposed to penta-BDE was slightly increased, and percentage macrophage in dams and PND21 exposed to deca-BDE was decreased. The ELISA results of antibody production show no significant difference in all treatment groups relative to controls.
CONCLUSION
These results imply that PBDEs given to the dam were transferred to the offspring during gestation and lactation, and PBDEs transferred from the dam affect immune system of offspring.
Keyword
MeSH Terms
-
Animals
Antibody Formation
Biphenyl Compounds
Body Weight
Cell Proliferation
Corn Oil
Enzyme-Linked Immunosorbent Assay
Ether, Ethyl
Female
Halogenated Diphenyl Ethers
Humans
Immune System
Lactation
Macrophages
Mice
Neutrophils
Phenyl Ethers
Pregnancy
Spleen
Thymocytes
Thymus Gland
Biphenyl Compounds
Corn Oil
Ether, Ethyl
Halogenated Diphenyl Ethers
Phenyl Ethers
Figure
Reference
-
1. Fernlöf G, Gadhasson I, Pödra K, Darnerud PO, Thuvander A. Lack of effects of some individual polybrominated diphenyl ether (PBDE) and polychlorinated biphenyl (PCB) congeners on human lymphocyte functions in vitro. Toxicol Lett. 1997. 90:189–197.
Article2. Darnerud PO, Thuvander A. Studies on immunological effects of polybrominated diphenyl ethers (PBDE) and polychlorinated biphenyls (PCB) exposure in rats and mice. Organohalogen Compounds. 1998. 35:415–418.3. Fowles JR, Fairbrother A, Baecher-Steppan L, Kerkvliet NI. Immunologic and endocrine effects of the flame-retardant pentabromodiphenyl ether (DE-71) in C57BL/6J mice. Toxicology. 1994. 86:49–61.
Article4. Martin PA, Mayne GJ, Bursian FS, Tomy G, Palace V, Pekarik C, Smits J. Immunotoxicity of the commercial polybrominated diphenyl ether mixture DE-71 in ranch mink (mustela vison). Environ Toxicol Chem. 2007. 26:988–997.
Article5. Arena SM, Greeley EH, Halbrook RS, Hansen LG, Segre M. Biological effects of gestational and lactational PCB exposure in neonatal and juvenile C57BL/6 mice. Arch Environ Contam Toxicol. 2003. 44:272–280.
Article6. Daniel V, Huber W, Bauer K, Suesal C, Conradt C, Opelz G. Associations of blood levels of PCB, HCHS, and HCB with numbers of lymphocyte subpopulations, in vitro lymphocyte response, plasma cytokine levels, and immunoglobulin autoantibodies. Environ Health Perspect. 2001. 109:173–178.
Article7. Stack AS, Altman-Hamamdzic S, Morris PJ, London SD, London L. Polychlorinated biphenyl mixtures (Aroclors) inhibit LPS-induced murine splenocyte proliferation in vitro. Toxicology. 1999. 139:137–154.
Article8. Tryphonas H, Luster MI, Schiffman G, Dawson LL, Hodgen M, Germolec D, Hayward S, Bryce F, Loo JC, Mandy F, et al. Effect of chronic exposure of PCB (Aroclor 1254) on specific and nonspecific immune parameters in the rhesus (Macaca mulatta) monkey. Fundam Appl Toxicol. 1991. 16:773–786.
Article9. Yoo BS, Jung KH, Hana SB, Kim HM. Apoptosis-mediated immunotoxicity of polychlorinated biphenyls (PCBs) in murine splenocytes. Toxicol Lett. 1997. 91:83–89.
Article10. Watanabe I, Sakai S. Environmental release and behavior of brominated flame retardants--an overview. Organohalogen Compounds. 2001. 52:1–4.11. European Union (EU). PE-CONS 3662/02. Official Journal C 127E - Directive of the European Parliament and of the Council on the restriction of the use of certain hazardous substances in electrical and electronic equipment, 2002. Brussels: 510–515.12. Tullo A. Great Lakes to phase out two flame retardants. Chem Eng News. 2003. 81:13.13. Darnerud PO. Toxic effects of brominated flame retardants in man and in wildlife. Environ Int. 2003. 29:841–853.
Article14. Schecter A, Pavuk M, Papke O, Ryan JJ, Birnbaum L, Rosen R. Polybrominated diphenyl ethers (PBDEs) in US mothers' milk. Enviro Health Perspect. 2003. 111:1723–1729.
Article15. Fernie KJ, Mayne G, Shutt JL, Pekarik C, Grasman KA, Letcher RJ, Drouillard K. Evidence of immunomodulation in nestling American kestrels (Falco sparverius) exposed to environmentally relevant PBDEs. Environ Pollut. 2005. 138:485–493.
Article16. Richardson VM, Staskal DF, Ross DG, Diliberto JJ, DeVito MJ, Birnbaum LS. Possible mechanisms of thyroid hormone disruption in mice by BDE 47, a major polybrominated diphenyl ether congener. Toxicol Appl Pharmacol. 2008. 226:244–250.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Depositional characteristics of atmospheric polybrominated diphenyl ethers on tree barks
- Prenatal Exposures to Environmental Chemicals and Children's Neurodevelopment: An Update
- Failure of immunization with Naegleria fowleri in mice born to immune mothers
- Effect of feed restriction during gestation and lactation period on changes in organ weight in rat offspring
- The effect of active immunization with Acanthamoeba culbertsoni in mice born to immune mother