Korean J Physiol Pharmacol.
2016 Jan;20(1):111-117. 10.4196/kjpp.2016.20.1.111.
Inducibility of human atrial fibrillation in an in silico model reflecting local acetylcholine distribution and concentration
- Affiliations
-
- 1Division of Cardiology, Yonsei University Health System, Seoul 03722, Korea. hnpak@yuhs.ac
- 2Department of Mechanical and Biomedical Engineering, Kangwon National University, Chuncheon 24341, Korea. ebshim@kangwon.ac.kr
- KMID: 2150480
- DOI: http://doi.org/10.4196/kjpp.2016.20.1.111
Abstract
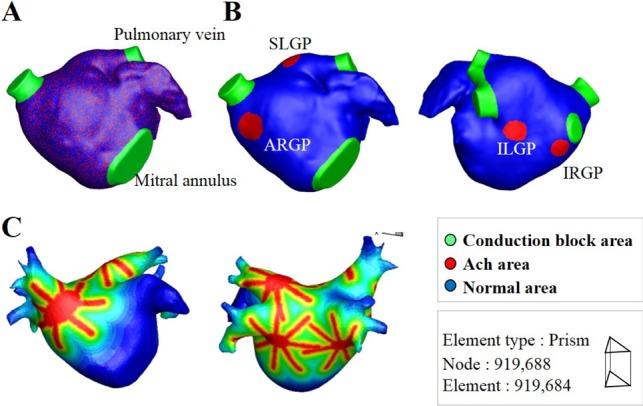
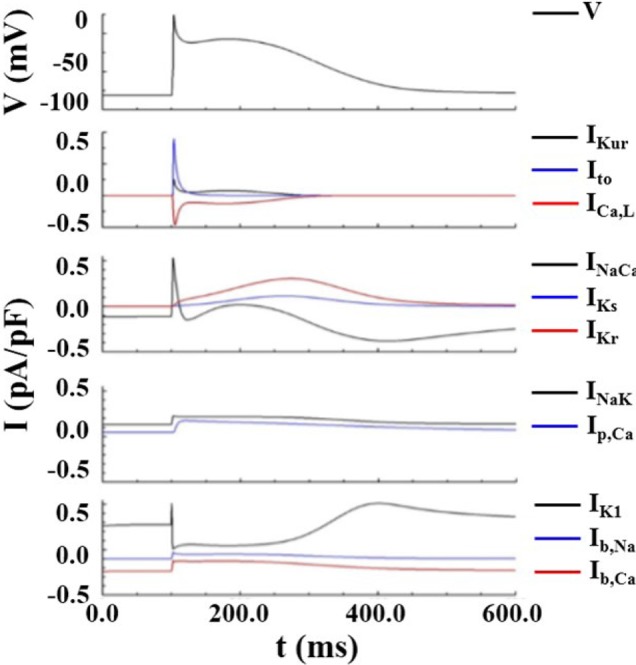
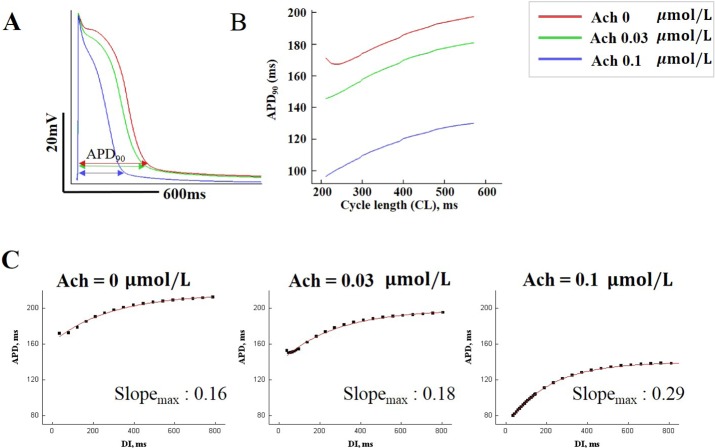
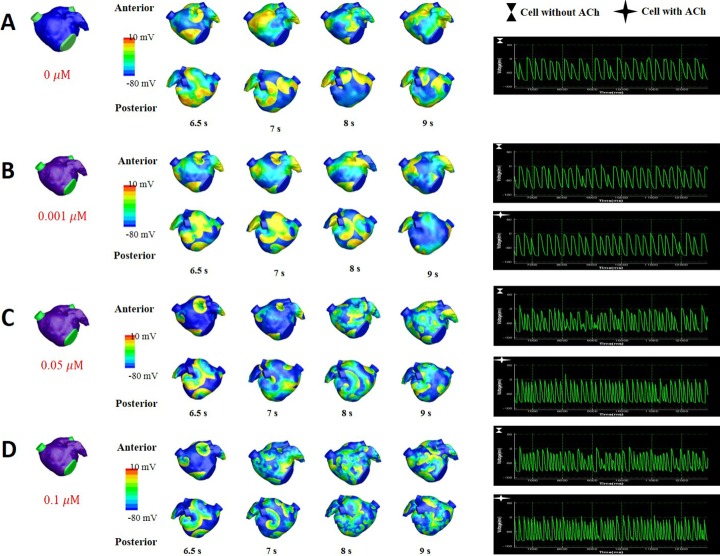
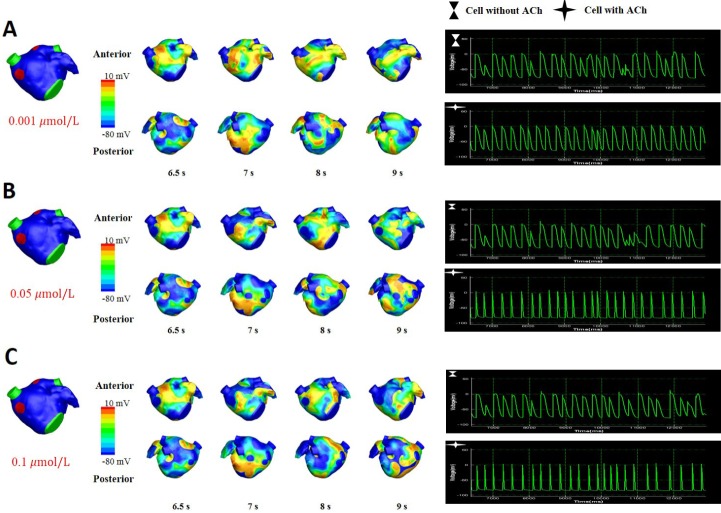
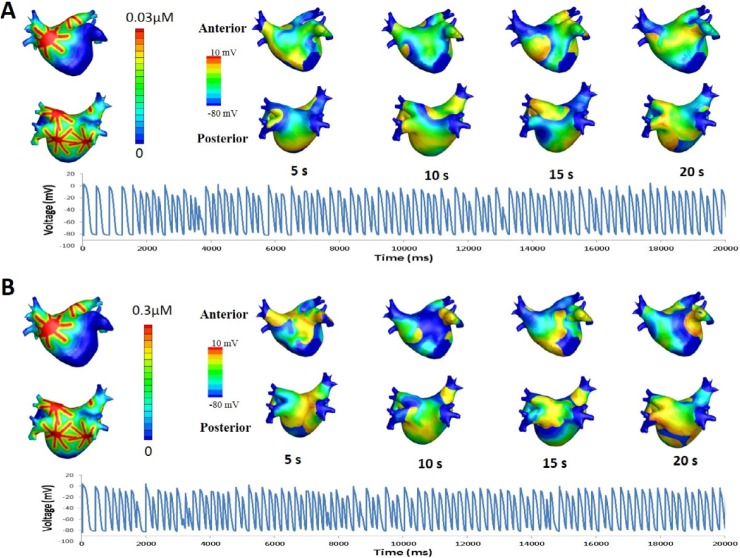
- Vagal nerve activity has been known to play a crucial role in the induction and maintenance of atrial fibrillation (AF). However, it is unclear how the distribution and concentration of local acetylcholine (ACh) promotes AF. In this study, we investigated the effect of the spatial distribution and concentration of ACh on fibrillation patterns in an in silico human atrial model. A human atrial action potential model with an ACh-dependent K+ current (I(KAch)) was used to examine the effect of vagal activation. A simulation of cardiac wave dynamics was performed in a realistic 3D model of the atrium. A model of the ganglionated plexus (GP) and nerve was developed based on the "octopus hypothesis". The pattern of cardiac wave dynamics was examined by applying vagal activation to the GP areas or randomly. AF inducibility in the octopus hypothesis-based GP and nerve model was tested. The effect of the ACh concentration level was also examined. In the single cell simulation, an increase in the ACh concentration shortened APD90 and increased the maximal slope of the restitution curve. In the 3D simulation, a random distribution of vagal activation promoted wavebreaks while ACh secretion limited to the GP areas did not induce a noticeable change in wave dynamics. The octopus hypothesis-based model of the GP and nerve exhibited AF inducibility at higher ACh concentrations. In conclusion, a 3D in silico model of the GP and parasympathetic nerve based on the octopus model exhibited higher AF inducibility with higher ACh concentrations.
MeSH Terms
Figure
Reference
-
1. Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG, Hinchey JA, Howard VJ, Jauch EC, Levine SR, Meschia JF, Moore WS, Nixon JV, Pearson TA. American Heart Association Stroke Council. Council on Cardiovascular Nursing. Council on Epidemiology and Prevention. Council for High Blood Pressure Research,. Council on Peripheral Vascular Disease, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011; 42:517–584. PMID: 21127304.2. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001; 285:2370–2375. PMID: 11343485.3. Ball J, Carrington MJ, McMurray JJ, Stewart S. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol. 2013; 167:1807–1824. PMID: 23380698.
Article4. Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014; 114:1500–1515. PMID: 24763467.5. Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol. 1998; 275:H301–H321. PMID: 9688927.
Article6. Kneller J, Zou R, Vigmond EJ, Wang Z, Leon LJ, Nattel S. Cholinergic atrial fibrillation in a computer model of a two-dimensional sheet of canine atrial cells with realistic ionic properties. Circ Res. 2002; 90:E73–E87. PMID: 12016272.
Article7. Kim BS, Kim YH, Hwang GS, Pak HN, Lee SC, Shim WJ, Oh DJ, Ro YM. Action potential duration restitution kinetics in human atrial fibrillation. J Am Coll Cardiol. 2002; 39:1329–1336. PMID: 11955851.
Article8. Zhou J, Scherlag BJ, Edwards J, Jackman WM, Lazzara R, Po SS. Gradients of atrial refractoriness and inducibility of atrial fibrillation due to stimulation of ganglionated plexi. J Cardiovasc Electrophysiol. 2007; 18:83–90. PMID: 17229305.
Article9. Katritsis DG, Giazitzoglou E, Zografos T, Pokushalov E, Po SS, Camm AJ. Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm. 2011; 8:672–678. PMID: 21199686.
Article10. Garfinkel A, Kim YH, Voroshilovsky O, Qu Z, Kil JR, Lee MH, Karagueuzian HS, Weiss JN, Chen PS. Preventing ventricular fibrillation by flattening cardiac restitution. Proc Natl Acad Sci U S A. 2000; 97:6061–6066. PMID: 10811880.
Article11. Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995; 92:1954–1968. PMID: 7671380.12. Lee AM, Aziz A, Didesch J, Clark KL, Schuessler RB, Damiano RJ Jr. Importance of atrial surface area and refractory period in sustaining atrial fibrillation: testing the critical mass hypothesis. J Thorac Cardiovasc Surg. 2013; 146:593–598. PMID: 22995722.
Article13. Scanavacca M, Pisani CF, Hachul D, Lara S, Hardy C, Darrieux F, Trombetta I, Negrão CE, Sosa E. Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Circulation. 2006; 114:876–885. PMID: 16923757.
Article14. Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec. 2000; 259:353–382. PMID: 10903529.
Article15. Chevalier P, Tabib A, Meyronnet D, Chalabreysse L, Restier L, Ludman V, Aliès A, Adeleine P, Thivolet F, Burri H, Loire R, François L, Fanton L. Quantitative study of nerves of the human left atrium. Heart Rhythm. 2005; 2:518–522. PMID: 15840477.
Article16. Ninomiya I. Direct evidence of nonuniform distribution of vagal effects on dog atria. Circ Res. 1966; 19:576–583. PMID: 5925156.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical significance of serum TSH in euthyroid patients with paroxysmal atrial fibrillation
- Pathophysiology and Diagnosis in Atrial Fibrillation
- Pharmacological Treatment of Atrial Fibrillation
- Non-medication Treatment of Atrial Fibrillation
- The Mechanism of and Preventive Therapy for Stroke in Patients with Atrial Fibrillation