Korean J Physiol Pharmacol.
2016 Jan;20(1):1-8. 10.4196/kjpp.2016.20.1.1.
Neural circuit remodeling and structural plasticity in the cortex during chronic pain
- Affiliations
-
- 1Department of Physiology, College of Korean Medicine, Kyung Hee University, Seoul 02447, Korea. skkim77@khu.ac.kr
- KMID: 2150467
- DOI: http://doi.org/10.4196/kjpp.2016.20.1.1
Abstract
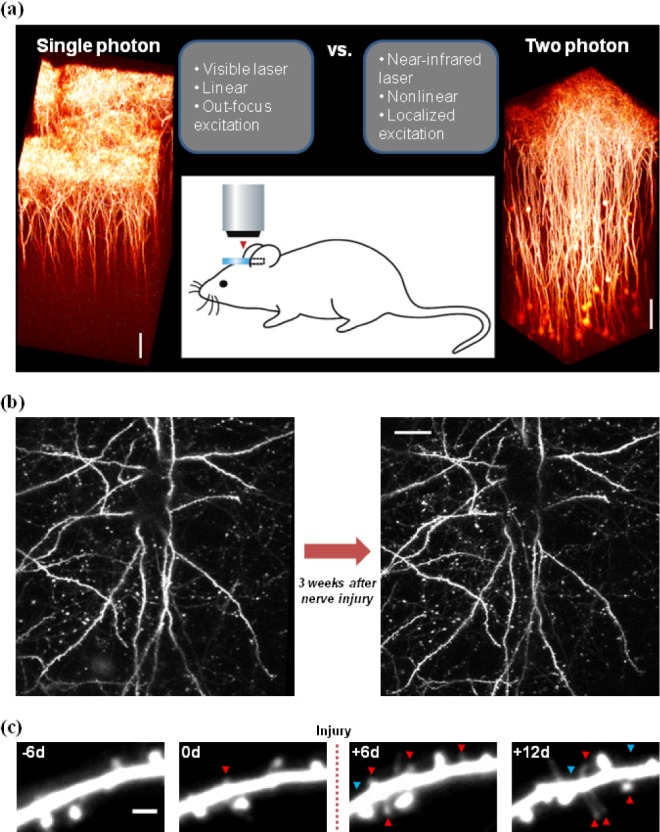
- Damage in the periphery or spinal cord induces maladaptive plastic changes along the somatosensory nervous system from the periphery to the cortex, often leading to chronic pain. Although the role of neural circuit remodeling and structural synaptic plasticity in the 'pain matrix' cortices in chronic pain has been thought as a secondary epiphenomenon to altered nociceptive signaling in the spinal cord, progress in whole brain imaging studies on human patients and animal models has suggested a possibility that plastic changes in cortical neural circuits may actively contribute to chronic pain symptoms. Furthermore, recent development in two-photon microscopy and fluorescence labeling techniques have enabled us to longitudinally trace the structural and functional changes in local circuits, single neurons and even individual synapses in the brain of living animals. These technical advances has started to reveal that cortical structural remodeling following tissue or nerve damage could rapidly occur within days, which are temporally correlated with functional plasticity of cortical circuits as well as the development and maintenance of chronic pain behavior, thereby modifying the previous concept that it takes much longer periods (e.g. months or years). In this review, we discuss the relation of neural circuit plasticity in the 'pain matrix' cortices, such as the anterior cingulate cortex, prefrontal cortex and primary somatosensory cortex, with chronic pain. We also introduce how to apply long-term in vivo two-photon imaging approaches for the study of pathophysiological mechanisms of chronic pain.
Keyword
MeSH Terms
Figure
Reference
-
1. Kuner R. Central mechanisms of pathological pain. Nat Med. 2010; 16:1258–1266. PMID: 20948531.
Article2. Saab CY. Pain-related changes in the brain: diagnostic and therapeutic potentials. Trends Neurosci. 2012; 35:629–637. PMID: 22763295.
Article3. Banic B, Petersen-Felix S, Andersen OK, Radanov BP, Villiger PM, Arendt-Nielsen L, Curatolo M. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain. 2004; 107:7–15. PMID: 14715383.
Article4. Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000; 288:1765–1769. PMID: 10846153.
Article5. Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009; 87:81–97. PMID: 18952143.
Article6. Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004; 431:782–788. PMID: 15483599.
Article7. Seminowicz DA, Laferriere AL, Millecamps M, Yu JS, Coderre TJ, Bushnell MC. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. Neuroimage. 2009; 47:1007–1014. PMID: 19497372.
Article8. May A. Chronic pain may change the structure of the brain. Pain. 2008; 137:7–15. PMID: 18410991.
Article9. Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003; 26:696–705. PMID: 14624855.
Article10. Proudlock F, Spike RC, Todd AJ. Immunocytochemical study of somatostatin, neurotensin, GABA, and glycine in rat spinal dorsal horn. J Comp Neurol. 1993; 327:289–297. PMID: 7678841.
Article11. Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992; 355:75–78. PMID: 1370574.
Article12. West SJ, Bannister K, Dickenson AH, Bennett DL. Circuitry and plasticity of the dorsal horn--toward a better understanding of neuropathic pain. Neuroscience. 2015; 300:254–275. PMID: 25987204.13. Zhang Y, Chen Y, Liedtke W, Wang F. Lack of evidence for ectopic sprouting of genetically labeled Aβ touch afferents in inflammatory and neuropathic trigeminal pain. Mol Pain. 2015; 11:18. PMID: 25880319.
Article14. Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009; 10:647–658. PMID: 19693029.
Article15. Tan AM, Chang YW, Zhao P, Hains BC, Waxman SG. Rac1-regulated dendritic spine remodeling contributes to neuropathic pain after peripheral nerve injury. Exp Neurol. 2011; 232:222–233. PMID: 21963650.
Article16. Tan AM, Samad OA, Fischer TZ, Zhao P, Persson AK, Waxman SG. Maladaptive dendritic spine remodeling contributes to diabetic neuropathic pain. J Neurosci. 2012; 32:6795–6807. PMID: 22593049.
Article17. Tan AM, Choi JS, Waxman SG, Hains BC. Dendritic spine remodeling after spinal cord injury alters neuronal signal processing. J Neurophysiol. 2009; 102:2396–2409. PMID: 19692517.
Article18. Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004; 24:10410–10415. PMID: 15548656.
Article19. Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007; 27:4004–4007. PMID: 17428976.
Article20. Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008; 60:570–581. PMID: 19038215.
Article21. Schmidt-Wilcke T, Leinisch E, Straube A, Kämpfe N, Draganski B, Diener HC, Bogdahn U, May A. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005; 65:1483–1486. PMID: 16275843.
Article22. Eto K, Wake H, Watanabe M, Ishibashi H, Noda M, Yanagawa Y, Nabekura J. Inter-regional contribution of enhanced activity of the primary somatosensory cortex to the anterior cingulate cortex accelerates chronic pain behavior. J Neurosci. 2011; 31:7631–7636. PMID: 21613476.
Article23. Kim SK, Eto K, Nabekura J. Synaptic structure and function in the mouse somatosensory cortex during chronic pain: in vivo two-photon imaging. Neural Plast. 2012; 2012:640259. PMID: 22530157.24. Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005; 9:463–484. PMID: 15979027.
Article25. Lithwick A, Lev S, Binshtok AM. Chronic pain-related remodeling of cerebral cortex - 'pain memory': a possible target for treatment of chronic pain. Pain Manag. 2013; 3:35–45. PMID: 24645930.
Article26. Takeuchi Y, Yamasaki M, Nagumo Y, Imoto K, Watanabe M, Miyata M. Rewiring of afferent fibers in the somatosensory thalamus of mice caused by peripheral sensory nerve transection. J Neurosci. 2012; 32:6917–6930. PMID: 22593060.
Article27. Draganski B, Moser T, Lummel N, Gãnssbauer S, Bogdahn U, Haas F, May A. Decrease of thalamic gray matter following limb amputation. Neuroimage. 2006; 31:951–957. PMID: 16520065.
Article28. MacIver K, Lloyd DM, Kelly S, Roberts N, Nurmikko T. Phantom limb pain, cortical reorganization and the therapeutic effect of mental imagery. Brain. 2008; 131:2181–2191. PMID: 18567624.
Article29. Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009; 10:59–70. PMID: 19096369.30. Baliki MN, Baria AT, Apkarian AV. The cortical rhythms of chronic back pain. J Neurosci. 2011; 31:13981–13990. PMID: 21957259.
Article31. Kim BG, Dai HN, McAtee M, Vicini S, Bregman BS. Remodeling of synaptic structures in the motor cortex following spinal cord injury. Exp Neurol. 2006; 198:401–415. PMID: 16443221.
Article32. Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001; 6:13–34. PMID: 11244481.
Article33. Manning BH, Merin NM, Meng ID, Amaral DG. Reduction in opioid- and cannabinoid-induced antinociception in rhesus monkeys after bilateral lesions of the amygdaloid complex. J Neurosci. 2001; 21:8238–8246. PMID: 11588195.
Article34. Rhudy JL, Meagher MW. Negative affect: effects on an evaluative measure of human pain. Pain. 2003; 104:617–626. PMID: 12927634.
Article35. Tajerian M, Leu D, Zou Y, Sahbaie P, Li W, Khan H, Hsu V, Kingery W, Huang TT, Becerra L, Clark JD. Brain neuroplastic changes accompany anxiety and memory deficits in a model of complex regional pain syndrome. Anesthesiology. 2014; 121:852–865. PMID: 25093591.
Article36. Gonçalves L, Silva R, Pinto-Ribeiro F, Pêgo JM, Bessa JM, Pertovaara A, Sousa N, Almeida A. Neuropathic pain is associated with depressive behaviour and induces neuroplasticity in the amygdala of the rat. Exp Neurol. 2008; 213:48–56. PMID: 18599044.
Article37. Liu MG, Chen J. Preclinical research on pain comorbidity with affective disorders and cognitive deficits: Challenges and perspectives. Prog Neurobiol. 2014; 116:13–32. PMID: 24444673.
Article38. Zhuo M. Cortical excitation and chronic pain. Trends Neurosci. 2008; 31:199–207. PMID: 18329111.
Article39. Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW, Zhuo M. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci. 2008; 28:7445–7453. PMID: 18632948.
Article40. Blom SM, Pfister JP, Santello M, Senn W, Nevian T. Nerve injury-induced neuropathic pain causes disinhibition of the anterior cingulate cortex. J Neurosci. 2014; 34:5754–5764. PMID: 24760836.
Article41. Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004; 303:1162–1167. PMID: 14976306.
Article42. Duric V, McCarson KE. Persistent pain produces stress-like alterations in hippocampal neurogenesis and gene expression. J Pain. 2006; 7:544–555. PMID: 16885011.
Article43. Zimmerman ME, Pan JW, Hetherington HP, Lipton ML, Baigi K, Lipton RB. Hippocampal correlates of pain in healthy elderly adults: a pilot study. Neurology. 2009; 73:1567–1570. PMID: 19901248.
Article44. Terada M, Kuzumaki N, Hareyama N, Imai S, Niikura K, Narita M, Yamazaki M, Suzuki T, Narita M. Suppression of enriched environment-induced neurogenesis in a rodent model of neuropathic pain. Neurosci Lett. 2008; 440:314–318. PMID: 18565655.
Article45. Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, Xin WJ, Wei XH, Wang J, Zhu HQ, Wu CY, Qin ZH, Liu G, Liu XG. Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-α in rodents. Neuropsychopharmacology. 2011; 36:979–992. PMID: 21289602.
Article46. Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Radulovic J, Martina M, Miller RJ, Apkarian AV. Abnormalities in hippocampal functioning with persistent pain. J Neurosci. 2012; 32:5747–5756. PMID: 22539837.
Article47. Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001; 98:4259–4264. PMID: 11259662.
Article48. Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci U S A. 2009; 106:2423–2428. PMID: 19171885.
Article49. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009; 32:1–32. PMID: 19400724.50. Seifert F, Maihöfner C. Central mechanisms of experimental and chronic neuropathic pain: findings from functional imaging studies. Cell Mol Life Sci. 2009; 66:375–390. PMID: 18791842.
Article51. Florence SL, Taub HB, Kaas JH. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science. 1998; 282:1117–1121. PMID: 9804549.
Article52. Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984; 224:591–605. PMID: 6725633.
Article53. Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002; 420:788–794. PMID: 12490942.
Article54. De Paola V, Holtmaat A, Knott G, Song S, Wilbrecht L, Caroni P, Svoboda K. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006; 49:861–875. PMID: 16543134.
Article55. Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol. 2009; 71:261–282. PMID: 19575680.
Article56. Kim SK, Nabekura J. Rapid synaptic remodeling in the adult somatosensory cortex following peripheral nerve injury and its association with neuropathic pain. J Neurosci. 2011; 31:5477–5482. PMID: 21471384.
Article57. Kim SK, Kato G, Ishikawa T, Nabekura J. Phase-specific plasticity of synaptic structures in the somatosensory cortex of living mice during neuropathic pain. Mol Pain. 2011; 7:87. PMID: 22067412.
Article58. Nemoto T. Living cell functions and morphology revealed by two-photon microscopy in intact neural and secretory organs. Mol Cells. 2008; 26:113–120. PMID: 18594180.59. Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol. 2003; 21:1369–1377. PMID: 14595365.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Three Musketeers in the Medial Prefrontal Cortex: Subregion-specific Structural and Functional Plasticity Underlying Fear Memory Stages
- Altered synaptic connections and inhibitory network of the primary somatosensory cortex in chronic pain
- The New Neurobiology of Depression
- Neuroplasticity in chronic pain: insights into diagnosis and treatment
- Imipramine Ameliorates Depressive Symptoms by Blocking Differential Alteration of Dendritic Spine Structure in Amygdala and Prefrontal Cortex of Chronic Stress-Induced Mice