Chonnam Med J.
2010 Dec;46(3):140-147. 10.4068/cmj.2010.46.3.140.
Anti-Inflammatory Activity of Houttuynia cordata against Lipoteichoic Acid-Induced Inflammation in Human Dermal Fibroblasts
- Affiliations
-
- 1Department of Dermatology, Chonnam National University Medical School, Gwangju, Korea. schul@chonnam.ac.kr
- 2Department of Dermatology, Chonnam National University Hospital, Research Institute of Clinical Medicine, Gwangju, Korea.
- KMID: 2116767
- DOI: http://doi.org/10.4068/cmj.2010.46.3.140
Abstract
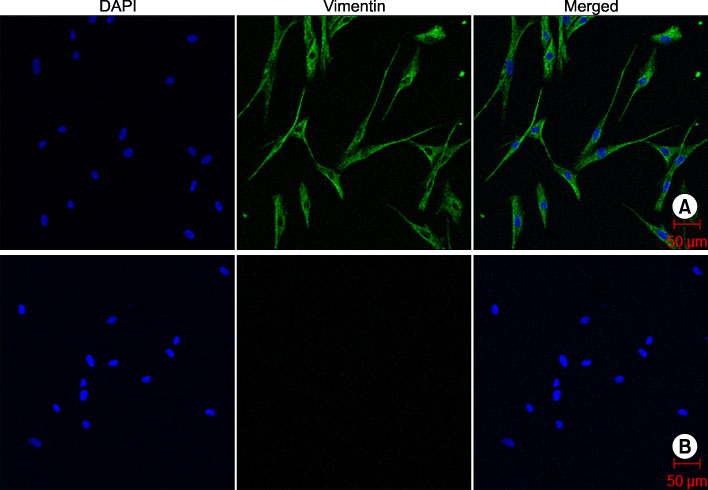
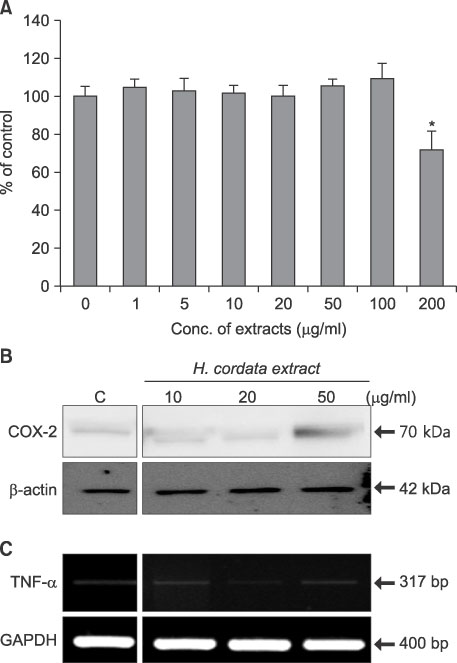
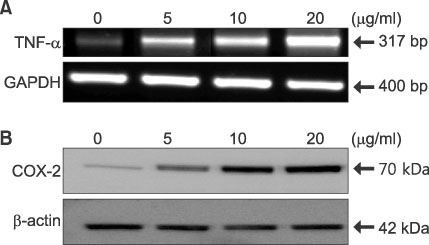
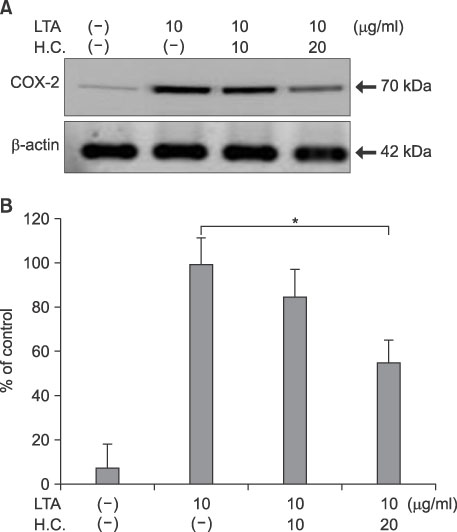
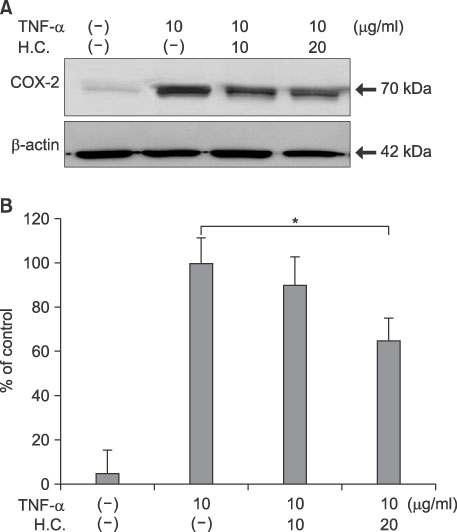
- Houttuynia cordata (H. cordata) has anti-inflammatory and anti-bacterial activities. Staphylococcus aureus (S. aureus)-producing lipoteichoic acid (LTA) has been implicated in inflammatory and immunologic responses in patients with atopic dermatitis (AD). The purpose of this study was to determine the potential of H. cordata as a novel anti-inflammatory agent for AD by evaluating the anti-inflammatory activity of H. cordata water extract on LTA-induced inflammation in dermal fibroblasts (DFs). The anti-inflammatory activity of H. cordata was evaluated by studying the inflammatory markers tumor necrosis factor-alpha (TNF-alpha) and cyclooxygenase-2 (COX-2). As shown by MTT assay and COX-2 immunoblot, H. cordata was not toxic to DFs in doses up to 20microg/ml. In RT-PCR and Western blot analysis, LTA up-regulated TNF-alpha (mRNA) and COX-2 (protein) at doses of 5~20microg/ml in a dose-dependent manner. Next, DFs were treated with LTA (10microg/ml) in the absence (control) or presence of H. cordata for 24 h. The level of LTA-induced TNF-alpha mRNA was suppressed by H. cordata (20microg/ml) by up to 40% compared with the level in LTA-treated control samples. Under the same conditions, the level of LTA-induced COX-2 protein was suppressed by H. cordata treatment by up to 52% compared with the level in LTA-treated control samples. When DFs were treated with TNF-alpha (10microg/ml) for 24 h, TNF-alpha-induced COX-2 expression was down-regulated by H. cordata treatment to 65% of that in control samples. In conclusion, H. cordata has anti-inflammatory activity against LTA-induced inflammation in DFs, in part by blocking the TNF-alpha pathway.
Keyword
MeSH Terms
-
Blotting, Western
Cyclooxygenase 2
Dermatitis, Atopic
Drugs, Chinese Herbal
Fibroblasts
Houttuynia
Humans
Inflammation
Lipopolysaccharides
RNA, Messenger
Staphylococcus aureus
Teichoic Acids
Tumor Necrosis Factor-alpha
Water
Cyclooxygenase 2
Drugs, Chinese Herbal
Lipopolysaccharides
RNA, Messenger
Teichoic Acids
Tumor Necrosis Factor-alpha
Water
Figure
Reference
-
1. Lu HM, Liang YZ, Yi LZ, Wu XJ. Anti-inflammatory effect of Houttuynia cordata injection. J Ethnopharmacol. 2006. 104:245–249.2. Schnopp C, Ring J, Mempel M. The role of antibacterial therapy in atopic eczema. Expert Opin Pharmacother. 2010. 11:929–936.
Article3. Ginsburg I. Role of lipoteichoic acid in infection and inflammation. Lancet Infect Dis. 2002. 2:171–179.
Article4. Xia G, Kohler T, Peschel A. The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus. Int J Med Microbiol. 2010. 300:148–154.
Article5. Matsui K, Nishikawa A. Lipoteichoic acid from Staphylococcus aureus enhances allergen-specific immunoglobulin E production in mice. Clin Exp Allergy. 2003. 33:842–848.
Article6. Numerof RP, Asadullah K. Cytokine and anti-cytokine therapies for psoriasis and atopic dermatitis. BioDrugs. 2006. 20:93–103.
Article7. Albanesi C. Keratinocyte in allergic skin diseases. Curr Opin Allergy Clin Immunol. 2010. 10:452–456.8. Chaly YV, Selvan RS, Fegeding KV, Kolesnikova TS, Voitenok NN. Expression of IL-8 gene in human monocytes and lymphocytes: differential regulation by TNF and IL-1. Cytokine. 2000. 12:636–643.
Article9. Aufiero B, Guo M, Young C, Duanmu Z, Talwar H, Lee HK, et al. Staphylococcus aureus induces the expression of tumor necrosis factor-alpha in primary human keratinocytes. Int J Dermatol. 2007. 46:687–694.
Article10. Bashir MM, Sharma MR, Werth VP. TNF-alpha production in the skin. Arch Dermatol Res. 2009. 301:87–91.11. De Kossodo S, Cruz PD Jr, Dougherty I, Thompson P, Silva-Valdez M, Beutler B. Expression of the tumor necrosis factor gene by dermal fibroblasts in response to ultraviolet irradiation or lipopolysaccharide. J Invest Dermatol. 1995. 104:318–322.
Article12. Choi JY, Piao MS, Lee JB, Oh JS, Kim IG, Lee SC. Propionibacterium acnes stimulates pro-matrix metalloproteinase-2 expression through tumor necrosis factor-alpha in human dermal fibroblasts. J Invest Dermatol. 2008. 128:846–854.
Article13. Lee JL, Mukhtar H, Bickers DR, Kopelovich L, Athar M. Cyclooxygenases in the skin: pharmacological and toxicological implications. Toxicol Appl Pharmacol. 2003. 192:294–306.
Article14. Baroni A, Perfetto B, Ruocco E, Rossano F. Lipoteichoic acid and protein-A from Staphylococcus aureus stimulate release of hepatocyte growth factor (HGF) by human dermal fibroblasts. Arch Dermatol Res. 1998. 290:211–214.
Article15. Perfetto B, Donnarumma G, Criscuolo D, Paoletti I, Grimaldi E, Tufano MA, et al. Bacterial components induce cytokine and intercellular adhesion molecules-1 and activate transcription factors in dermal fibroblasts. Res Microbiol. 2003. 154:337–344.
Article16. Hayashi K, Kamiya M, Hayashi T. Virucidal effects of the steam distillate from Houttuynia cordata and its components on HSV-1, influenza virus, and HIV. Planta Med. 1995. 61:237–241.
Article17. Lu H, Wu X, Liang Y, Zhang J. Variation in chemical composition and antibacterial activities of essential oils from two species of Houttuynia Thumb. Chem Pharm Bull (Tokyo). 2006. 54:936–940.
Article18. Chomnawang MT, Surassmo S, Nukoolkarn VS, Gritsanapan W. Effect of Garcinia mangostana on inflammation caused by Propionibacterium acnes. Fitoterapia. 2007. 78:401–408.
Article19. Kim GS, Kim DH, Lim JJ, Lee JJ, Han DY, Lee WM, et al. Biological and antibacterial activities of the natural herb Houttuynia cordata water extract against the intracellular bacterial pathogen salmonella within the RAW 264.7 macrophage. Biol Pharm Bull. 2008. 31:2012–2017.
Article20. Kim SK, Ryu SY, No J, Choi SU, Kim YS. Cytotoxic alkaloids from Houttuynia cordata. Arch Pharm Res. 2001. 24:518–521.21. Chou SC, Su CR, Ku YC, Wu TS. The constituents and their bioactivities of Houttuynia cordata. Chem Pharm Bull (Tokyo). 2009. 57:1227–1230.22. Schmid-Grendelmeier P, Ballmer-Weber BK. Atopic dermatitis - current insights into path physiology and management. Ther Umsch. 2010. 67:175–185.23. Graham GM, Farrar MD, Cruse-Sawyer JE, Holland KT, Ingham E. Proinflammatory cytokine production by human keratinocytes stimulated with Propionibacterium acnes and P. acnes GroEL. Br J Dermatol. 2004. 150:421–428.
Article24. Ellingsen E, Morath S, Flo T, Schromm A, Hartung T, Thiemermann C, et al. Induction of cytokine production in human T cells and monocytes by highly purified lipoteichoic acid: involvement of Toll-like receptors and CD14. Med Sci Monit. 2002. 8:BR149–BR156.25. Fujii E, Irie K, Ogawa A, Ohba K, Muraki T. Role of nitric oxide and prostaglandins in lipopolysaccharide-induced increase in vascular permeability in mouse skin. Eur J Pharmacol. 1996. 297:257–263.
Article26. Wang HQ, Smart RC. Overexpression of protein kinase C-alpha in the epidermis of transgenic mice results in striking alterations in phorbol ester-induced inflammation and COX-2, MIP-2 and TNF-alpha expression but not tumor promotion. J Cell Sci. 1999. 112:3497–3506.
Article27. Athar M, An KP, Morel KD, Kim AL, Aszterbaum M, Longley J, et al. Ultraviolet B (UVB)-induced cox-2 expression in murine skin: an immunohistochemical study. Biochem Biophys Res Commun. 2001. 280:1042–1047.
Article28. von Aulock S, Schröder NW, Traub S, Gueinzius K, Lorenz E, Hartung T, et al. Heterozygous toll-like receptor 2 polymorphism does not affect lipoteichoic acid-induced chemokine and inflammatory responses. Infect Immun. 2004. 72:1828–1831.
Article29. Fournier B, Philpott DJ. Recognition of Staphylococcus aureus by the innate immune system. Clin Microbiol Rev. 2005. 18:521–540.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lignans and Macrolides from the Leaves of Houttuynia cordata with Inhibitory Activity against NO Production in Murine Macrophage RAW 264.7 Cells
- Increased Flavonoid Compounds from Fermented Houttuynia cordata using Isolated Six of Bacillus from Traditionally Fermented Houttuynia cordata
- Anti-Inflammatory Effect of Houttuynia Cordata Thunb in Activated Raw 264.7 Cells Activated with Lipopolysaccharide and Interferon Gamma
- Anti-inflammatory effects of Houttuynia cordata supercritical extract in carrageenan-air pouch inflammation model
- Anti-nociceptive and Anti-inflammatory Effect of an Ethanol Extract Mixture of Vitis amurensis, Aralia cordata, and Glycyrrhizae radix