J Korean Surg Soc.
2010 Dec;79(6):421-427. 10.4174/jkss.2010.79.6.421.
The Wound Healing Effect of a Silk Fibroin Film on Cutaneous Burn of Hairless Mice
- Affiliations
-
- 1Department of Pathology, College of Medicine, Hallym University, Chuncheon, Korea.
- 2Department of Biochemistry, College of Medicine, Hallym University, Chuncheon, Korea.
- 3Department of Surgery, College of Medicine, Hallym University, Chuncheon, Korea. biogra@hallym.or.kr
- 4Department of Radiology, College of Medicine, Hallym University, Chuncheon, Korea.
- 5Division of Natural Science, SahmYook University, Seoul, Korea.
- 6FineCo Ltd., Chuncheon, Korea.
- KMID: 2096596
- DOI: http://doi.org/10.4174/jkss.2010.79.6.421
Abstract
- PURPOSE
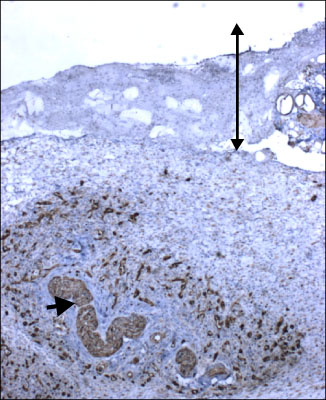
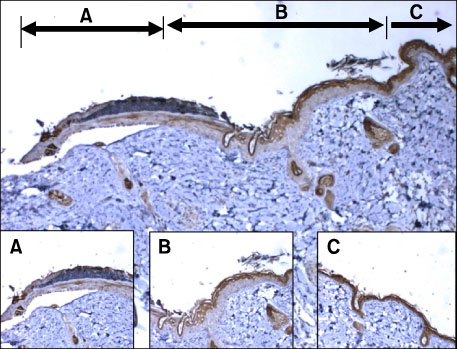
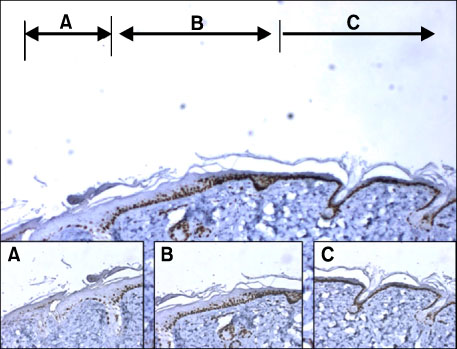
The aim of this study was to examine the effects of silk fibroin film on wound healing of cutaneous burn in hairless mice by using microscopic findings and stem cell markers (nestin, cytokeratin 15) and ki-67 proliferation marker.
METHODS
Each mouse received two burns at the dorsal area by applying a metal rod heated with boiling water. Burn wound sites were dressed with Silk Fibroin Film and duoderm (SF group), Aquacel hydrofiber and duoderm (AC group) and duoderm only (Control group). All groups were covered externally with duodermas adhesive bands. Those mice were sacrificed at zero, two, seven, fourteen and twenty one days after burn. Histological findings and immunohistochemical staining for stem cell markers were observed.
RESULTS
In SF group, inflammatory cell infiltration, formation of granulation tissue and inflammatory foci are greater than in AC and control group. Those factors appear to enhance mesenchymal stem cell markers such as nestin. Finally mesenchymal tissue regeneration was enhanced. In addition, the length of ki-67 expressed re-generating epithelium, which appeared to be associated with epithelial regeneration, was the longest in SF group.
CONCLUSION
The results show that the wound healing effect of SF is the best among other treatment materials including AC in the experimental group and duoderm in the control group through mesenchymal regeneration and epithelial regeneration which are essential factors for wound healing.
Keyword
MeSH Terms
-
Adhesives
Animals
Bandages, Hydrocolloid
Burns
Carboxymethylcellulose Sodium
Epithelium
Fibroins
Granulation Tissue
Hot Temperature
Intermediate Filament Proteins
Keratins
Mesenchymal Stromal Cells
Mice
Mice, Hairless
Nerve Tissue Proteins
Regeneration
Silk
Stem Cells
Water
Wound Healing
Adhesives
Carboxymethylcellulose Sodium
Fibroins
Intermediate Filament Proteins
Keratins
Nerve Tissue Proteins
Silk
Water
Figure
Reference
-
1. Korean Research Group for Wound Care. Advances in Wound Care. 2002. 1st ed. Seoul: Korea Medical Book Publisher;11–19. 167–195.2. Choi YH, Kim MG, Ahn DH, Cho SJ, Hong SH, Lee JY, et al. Immunohistochemical expression of stem cell markers during the wound healing process of cutaneous burn. J Korean Surg Soc. 2010. 79:1–7.3. Zhu H, Wei X, Bian K, Murad F. Effects of nitric oxide on skin burn wound healing. J Burn Care Res. 2008. 29:804–814.4. Yoon HJ, Shin JW, Kim YK, Kim JE, Cho KH. The effect of vitamin C and E on the expression of the cutaneous basement membrane components. Korean J Investig Dermatol. 2007. 14:87–98.5. Freddi G, Tsukada M, Beretta S. Structure and physical properties of silk fibroin/polyacrylamide blend films. J Appl Polym Sci. 1999. 71:1563–1571.6. Kato N, Sato S, Yamanaka A, Yamada H, Fuwa N, Nomura M. Silk protein, sericin, inhibits lipid peroxidation and tyrosinase activity. Biosci Biotechnol Biochem. 1998. 62:145–147.7. Sugihara A, Sugiura K, Morita H, Ninagawa T, Tubouchi K, Tobe R, et al. Promotive effects of a silk film on epidermal recovery from full-thickness skin wounds. Proc Soc Exp Biol Med. 2000. 225:58–64.8. Yamaguchi Y, Yoshikawa K. Cutaneous wound healing: an update. J Dermatol. 2001. 28:521–534.9. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003. 83:835–870.10. Suh W, Kim KL, Kim JM, Shin IS, Lee YS, Lee JY, et al. Transplantation of endothelial progenitor cells accelerates dermal wound healing with increased recruitment of monocytes/macrophages and neovascularization. Stem Cells. 2005. 23:1571–1578.11. Chiarini A, Petrini P, Bozzini S, Pra ID, Armato U. Silk fibroin/poly(carbonate)-urethane as a substrate for cell growth: in vitro interactions with human cells. Biomaterials. 2003. 24:789–799.12. Yeo JH, Lee KG, Lee YW. Collagen growth effects in rats using B.mori fibroin. Korean J Sericult Sci. 2001. 43:49–52.13. Sobajo C, Behzad F, Yuan XF, Bayat A. Silk: a potential medium for tissue engineering. Eplasty. 2008. 8:e47.14. Liu TL, Miao JC, Sheng WH, Xie YF, Huang Q, Shan YB, et al. Cytocompatibility of regenerated silk fibroin film: a medical biomaterial applicable to wound healing. J Zhejiang Univ Sci B. 2010. 11:10–16.15. Patel GK, Wilson CH, Harding KG, Finlay AY, Bowden PE. Numerous keratinocyte subtypes involved in wound reepithelialization. J Invest Dermatol. 2006. 126:497–502.16. Repesh LA, Oberpriller JC. Ultrastructural studies on migrating epidermal cells during the wound healing stage of regeneration in the adult newt, Notophthalmus viridescens. Am J Anat. 1980. 159:187–208.17. Jeong MJ. Wound healing and cell migration, trends in medical research cell organization. Korean Soc Med Biochem Mol Biol News. 2000. 7:20–26.18. Roh DH, Kang SY, Kim JY, Kwon YB, Kweon HY, Lee KG, et al. Wound healing effect of silk fibroin/alginate-blended sponge in full thickness skin defect of rat. J Mater Sci Mater Med. 2006. 17:547–552.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Silk Fibroin-Alginic Acid Sponge Treatment as an Artificial Skin on Wound
- Powdered Wound Dressing Materials Made from wild Silkworm Antheraea pernyi Silk Fibroin on Full-skin Thickness Burn Wounds on Rats
- Fabrication and Characterization of Hydrocolloid Dressing with Silk Fibroin Nanoparticles for Wound Healing
- Immunohistochemical Expression of Stem Cell Markers during the Wound Healing Process of Cutaneous Burn
- Effects of Topical Application of Halofuginone on Wound Healing