Korean Circ J.
2011 Sep;41(9):535-541. 10.4070/kcj.2011.41.9.535.
Effects of Ramiprilat-Coated Stents on Neointimal Hyperplasia, Inflammation, and Arterial Healing in a Porcine Coronary Restenosis Model
- Affiliations
-
- 1The Heart Center of Chonnam National University Hospital, Chonnam National University Research Institute of Medical Sciences, Gwangju, Korea. myungho@chollian.net
- 2Center for Functional Nano Fine Chemicals & School of Applied Chemical Engineering, Chonnam National University, Gwangju, Korea.
- KMID: 2094106
- DOI: http://doi.org/10.4070/kcj.2011.41.9.535
Abstract
- BACKGROUND AND OBJECTIVES
The renin-angiotensin-aldosterone system has been implicated in the pathogenesis of neointimal hyperplasia, and a role for angiotensin II in the migration and proliferation of vascular smooth muscle cells in restenotic lesions has been proposed. The aim of this study was to determine the anti-proliferative and anti-inflammatory effects of ramiprilat-coated stents in a porcine coronary overstretch restenosis model.
SUBJECTS AND METHODS
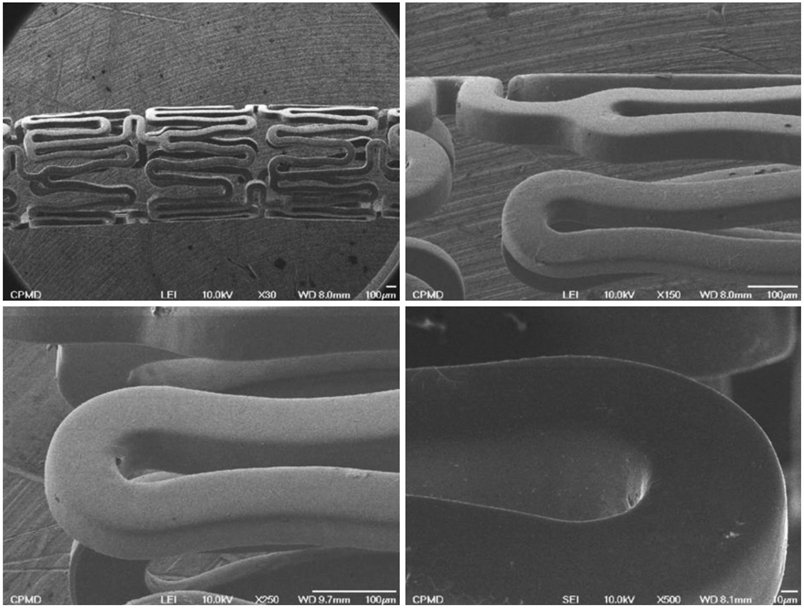
Pigs were randomized into two groups in which the coronary arteries {16 pigs (16 coronaries in each group)} had a 3.0x17 mm ramiprilat-coated MAC stent or a 3.0x17 mm control MAC stent (AMG, Munich, Germany) implanted with oversizing (stent-to-artery ratio, 1.3 : 1) in porcine coronary arteries, and histopathologic analysis was assessed 28 days after stenting.
RESULTS
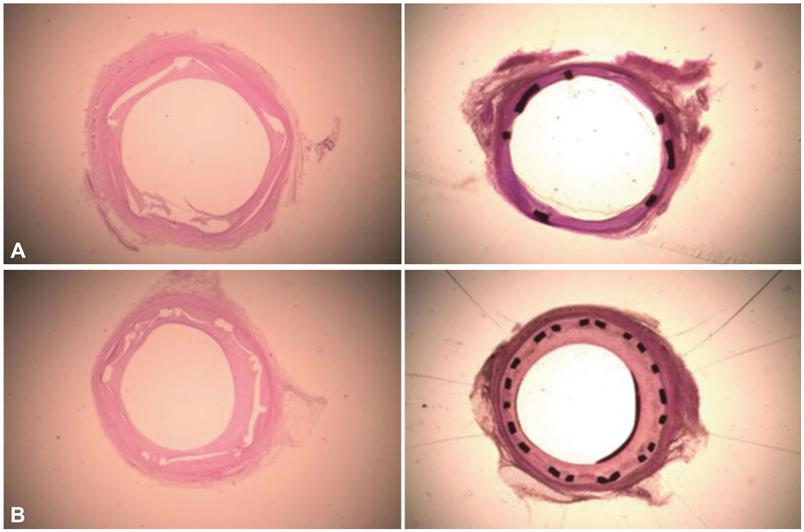
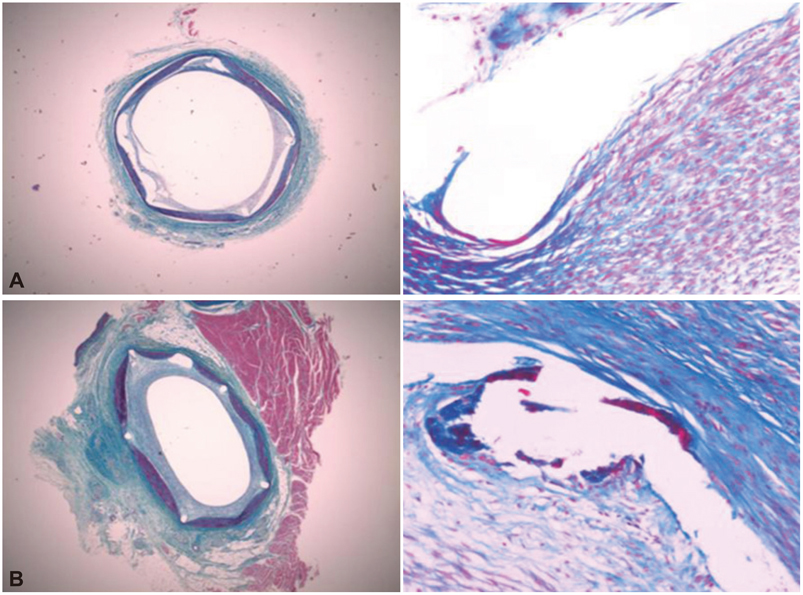
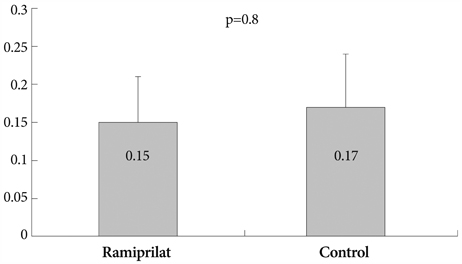
There were no significant differences in the injury and inflammation scores between the two groups (1.20+/-0.43 vs. 1.23+/-0.57, p=0.8; and 1.21+/-0.39 vs. 1.25+/-0.49, p=0.6, respectively). Within the neointima, most inflammatory cells were lymphohistiocytes. Significant positive correlations existed between inflammatory cell counts and the neointima areas (r=0.567, p<0.001), and between inflammatory cell counts and the percent area stenosis (r=0.478, p<0.001). There was no significant difference in the inflammatory cell counts normalized to the injury (110+/-89 vs. 123+/-83, p=0.4) and fibrin scores (0.15+/-0.06 vs. 0.17+/-0.07, p=0.8) between the 2 groups. There were trends toward a smaller neointima area (1.06+/-0.51 mm2 vs. 1.28+/-0.35 mm2, p=0.083) and a smaller percent area stenosis (18.9+/-8.7% vs. 21.8+/-7.2%, p=0.088) in the ramiprilat-coated stent group.
CONCLUSION
Although the ramiprilat-coated stent did not show significant inhibitory effects on neointimal hyperplasia, the ramiprilat-coated stent showed good effects on the inflammatory reaction and arterial healing similar to the control stent in a porcine coronary restenosis model.
MeSH Terms
Figure
Cited by 1 articles
-
Mechanical and Histopathological Comparison between Commercialized and Newly Designed Coronary Bare Metal Stents in a Porcine Coronary Restenosis Model
Kyung Seob Lim, In Ho Bae, Jung Ha Kim, Dae Sung Park, Jong Min Kim, Jung Hyun Kim, Doo Sun Sim, Young Joon Hong, Myung Ho Jeong
Chonnam Med J. 2013;49(1):7-13. doi: 10.4068/cmj.2013.49.1.7.
Reference
-
1. Forrester JS, Fishbein M, Helfant R, Fagin J. A paradigm for restenosis based on cell biology: clues for the development of new preventive therapies. J Am Coll Cardiol. 1991. 17:758–769.2. Austin GE, Ratliff NB, Hollman J, Tabei S, Phillips DF. Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1985. 6:369–375.3. Bae Y, Jeong MH, Jang YS, et al. Comparison of porcine coronary stent restenosis between MAC (Maximum Arterial Re-Creation) stent and Palmaz-Schatz stent. Korean Circ J. 1998. 28:89–96.4. Giraldo AA, Esposo OM, Meis JM. Intimal hyperplasia as a cause of restenosis after percutaneous transluminal coronary angioplasty. Arch Pathol Lab Med. 1985. 109:173–175.5. Ahn YK, Jeong MH, Kim JW, et al. Preventive effects of the heparin-coated stent on restenosis in the porcine model. Catheter Cardiovasc Interv. 1999. 48:324–330.6. Hong YJ, Jeong MH, Lim SY, et al. Elevated preprocedural high-sensitivity C-reactive protein levels are associated with neointimal hyperplasia and restenosis development after successful coronary artery stenting. Circ J. 2005. 69:1477–1483.7. Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB. Instent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol. 1998. 31:224–230.8. Grewe PH, Deneke T, Machraoui A, Barmeyer J, Müller KM. Acute and chronic tissue response to coronary stent implantation: pathologic findings in human specimen. J Am Coll Cardiol. 2000. 35:157–163.9. Farb A, Sangiorgi G, Carter AJ, et al. Pathology of acute and chronic coronary stenting in humans. Circulation. 1999. 99:44–52.10. Pratt RE, Dzau VJ. Pharmacological strategies to prevent restenosis: lessons learned from blockade of the renin-angiotensin system. Circulation. 1996. 93:848–852.11. Nakamura M, Funakoshi T, Arakawa N, Yoshida H, Makita S, Hiramori K. Effect of angiotensin-converting enzyme inhibitors on endothelium-dependent peripheral vasodilation in patients with chronic heart failure. J Am Coll Cardiol. 1994. 24:1321–1327.12. Hirooka Y, Imaizumi T, Masaki H, et al. Captopril improves impaired endothelium-dependent vasodilation in hypertensive patients. Hypertension. 1992. 20:175–180.13. Mancini GB, Henry GC, Macaya C, et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease: the TREND (Trial on Reversing Endothelial Dysfunction) Study. Circulation. 1996. 94:258–265.14. Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992. 19:267–274.15. Kolodgie FD, John M, Khurana C, et al. Sustained reduction of instent neointimal growth with the use of a novel systemic nanoparticle paclitaxel. Circulation. 2002. 106:1195–1198.16. Farb A, Sangiorgi G, Carter AJ, et al. Pathology of acute and chronic coronary stenting in humans. Circulation. 1999. 99:44–52.17. Finn AV, Kolodgie FD, Harnek J, et al. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus-or paclitaxel-eluting stents. Circulation. 2005. 112:270–278.18. Carter AJ, Aggarwal M, Kopia GA, et al. Long-term effects of polymer-based, slow-release, sirolimus-eluting stents in a porcine coronary model. Cardiovasc Res. 2004. 63:617–624.19. Farb A, Heller PF, Shroff S, et al. Pathological analysis of local delivery of paclitaxel via a polymer-coated stent. Circulation. 2001. 104:473–479.20. Ribichini F, Steffenino G, Dellavalle A, et al. Plasma activity and insertion/deletion polymorphism of angiotensin I-converting enzyme: a major risk factor and a marker of risk for coronary stent restenosis. Circulation. 1998. 97:147–154.21. Ribichini F, Vado A, Uslenghi E, Matullo G, Piazza A. Relationship between plasma ACE activity and the proliferative healing process in coronary vessel injury after coronary stenting. Atherosclerosis. 2000. 152:261–263.22. Ohishi M, Ueda M, Rakugi H, et al. Upregulation of angiotensin-converting enzyme during the healing process after injury at the site of percutaneous transluminal coronary angioplasty in humans. Circulation. 1997. 96:3328–3337.23. Bell L, Madri JA. Influence of the angiotensin system on endothelial and smooth muscle cell migration. Am J Pathol. 1990. 137:7–12.24. Pratt RE, Dzau VJ. Pharmacological strategies to prevent restenosis: lessons learned from blockade of the renin-angiotensin system. Circulation. 1996. 93:848–852.25. Campbell-Boswell M, Robertson AL Jr. Effects of angiotensin II and vasopressin on human smooth muscle cells in vitro. Exp Mol Pathol. 1981. 35:265–276.26. Daemen MJ, Lombardi DM, Bosman FT, Schwartz SM. Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ Res. 1991. 68:450–456.27. Scott-Burden T, Hahn AW, Resink TJ, Bühler FR. Modulation of extracellular matrix by angiotensin II: Stimulated glycoconjugate synthesis and growth in vascular smooth muscle cells. J Cardiovasc Pharmacol. 1990. 16:Suppl 4. S36–S41.28. Powell JS, Clozel JP, Muller RK, et al. Inhibitors of angiotensin-converting enzyme prevent myointimal proliferation after vascular injury. Science. 1989. 245:186–188.29. Huber KC, Schwartz RS, Edwards WD, et al. Effects of angiotensin converting enzyme inhibition on neointimal proliferation in a porcine coronary injury model. Am Heart J. 1993. 125:695–701.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The preventive effects of the heparin-coated coronary stent in a porcine coronary stent restenosis model
- Local Delivery of Nitric Oxide from an Eluting Stent to Inhibit Neointimal Thickening in a Porcine Coronary Injury Model
- The Effects of the Heparin-Coated Maximum Arterial Re-Creation (MAC) Stent on Porcine Coronary Stent Restenosis
- Anti-inflammatory Effect of Abciximab-Coated Stent in a Porcine Coronary Restenosis Model
- The effect of the carvedilol-loaded BiodivYsioTM DD stent on the inhibition of neointimal proliferation in a porcine coronary stent restenosis model