J Korean Med Sci.
2007 Oct;22(5):802-809. 10.3346/jkms.2007.22.5.802.
Anti-inflammatory Effect of Abciximab-Coated Stent in a Porcine Coronary Restenosis Model
- Affiliations
-
- 1The Heart Center of Chonnam National University Hospital, Chonnam National University Research Institute of Medical Sciences, Gwangju, Korea. myungho@chollian.net
- KMID: 1713284
- DOI: http://doi.org/10.3346/jkms.2007.22.5.802
Abstract
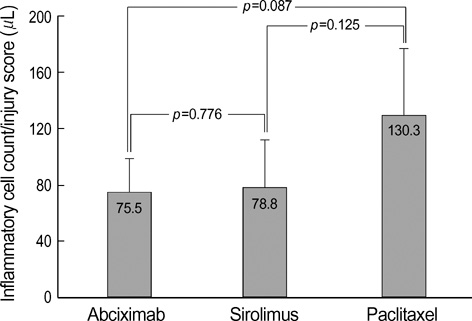
- The aim of this study was to examine the anti-inflammatory effect of abciximab-coated stent in a porcine coronary overstretch restenosis model. Ten abciximab-coated stents, ten sirolimus-eluting stents (SES), and ten paclitaxel-eluting stents (PES) were deployed with oversizing (stent/artery ratio 1.3:1) in porcine coronary arteries, and histopathologic analysis was done at 28 days after stenting. There were no significant differences in the neointima area normalized to injury score and inflammation score among the three stent groups (1.58+/-0.43 mm2, 1.57+/-0.39 mm2 in abciximab-coated stent group vs. 1.69+/-0.57 mm2, 1.72+/-0.49 mm2 in the SES group vs. 1.92+/-0.86 mm2, 1.79+/-0.87 mm2 in the PES group, respectively). In the neointima, most inflammatory cells were lymphohistiocytes. Significant positive correlations were found between the extent of inflammatory reaction and the neointima area (r=0.567, p<0.001) and percent area stenosis (r=0.587, p<0.001). Significant correlations were found between the injury score and neointimal area (r=0.645, p<0.001), between the injury score and the inflammation score (r=0.837, p<0.001), and between the inflammation score and neointimal area (r=0.536, p=0.001). There was no significant difference in the inflammatory cell counts normalized to injury score among the three stent groups (75.5+/-23.1/microliter in abciximabcoated stent group vs. 78.8+/-33.2/microliter in the SES group vs. 130.3+/-46.9/microliter in the PES group). Abciximab-coated stent showed comparable inhibition of inflammatory cell infiltration and neointimal hyperplasia with other drug-eluting stents in a porcine coronary restenosis model.
Keyword
MeSH Terms
-
Animals
Anti-Bacterial Agents/administration & dosage
Anti-Inflammatory Agents/*pharmacology
Antibodies, Monoclonal/administration & dosage/*pharmacology
Arteries/injuries/pathology
Constriction, Pathologic
Coronary Restenosis/*therapy
Disease Models, Animal
*Drug-Eluting Stents
Female
Hyperplasia
Immunoglobulin Fab Fragments/administration & dosage/*pharmacology
Inflammation
Paclitaxel/administration & dosage
Sirolimus/administration & dosage
Swine
Tunica Intima/pathology
Figure
Cited by 2 articles
-
Development of Novel Drug-Eluting Stents for Acute Myocardial Infarction
Doo Sun Sim, Myung Ho Jeong
Chonnam Med J. 2017;53(3):187-195. doi: 10.4068/cmj.2017.53.3.187.Preclinical Evaluation of a Novel Polymer-free Everolimus-eluting Stent in a Mid-term Porcine Coronary Restenosis Model
Kyung Hoon Cho, Myung Ho Jeong, Dae Sung Park, Moonki Kim, JungHa Kim, Jun-Kyu Park, Xiongyi Han, Dae Young Hyun, Min Chul Kim, Doo Sun Sim, Young Joon Hong, Ju Han Kim, Youngkeun Ahn
J Korean Med Sci. 2021;36(40):e259. doi: 10.3346/jkms.2021.36.e259.
Reference
-
1. Faxon DP, Spiro TE, Minor S, Cote G, Douglas J, Gottlieb R, Califf R, Dorosti K, Topol E, Gordon JB. Low molecular weight heparin in prevention of restenosis after angioplasty. Results of Enoxaparin Restenosis (ERA) Trial. Circulation. 1994. 90:908–914.
Article2. Hamm CW, Reimers J, Ischinger T, Rupprecht HJ, Berger J, Bleifeld W. German Angioplasty Bypass Surgery Investigation (GABI). A randomized study of coronary angioplasty compared with bypass surgery in patients with symptomatic multivessel coronary disease. N Engl J Med. 1994. 331:1037–1043.
Article3. Kent KM, Bentivoglio LG, Block PC, Bourassa MG, Cowley MJ, Dorros G, Detre KM, Gosselin AJ, Gruentzig AR, Kelsey SF. Long-term efficacy of percutaneous transluminal coronary angioplasty (PTCA): report from the National Heart, Lung, and Blood Institute PTCA Registry. Am J Cardiol. 1984. 53:27C–31C.
Article4. Forrester JS, Fishbein M, Helfant R, Fagin J. A paradigm for restenosis based on cell biology: clues for the development of new preventive therapies. J Am Coll Cardiol. 1991. 17:758–769.
Article5. Austin GE, Ratliff NB, Hollman J, Tabei S, Phillips DF. Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1985. 6:369–375.
Article6. Bae Y, Jeong MH, Ahn YK, Park JH, Cho JG, Park JC, Kang JC, Park OK. Comparison of porcine coronary stent restenosis between MAC (Maximal Arterial Re-Creation) stent and Palmaz-Schatz stent. Korean Circ J. 1998. 28:89–96.7. Giraldo AA, Esposo OM, Meis JM. Intimal hyperplasia as a cause of restenosis after percutaneous transluminal coronary angioplasty. Arch Pathol Lab Med. 1985. 109:173–175.8. Ahn YK, Jeong MH, Kim JW, Kim SH, Cho JH, Cho JG, Park CS, Juhng SW, Park JC, Kang JC. Preventive effects of the heparin-coated stent on restenosis in the porcine model. Catheter Cardiovasc Interv. 1999. 48:324–330.
Article9. Hong YJ, Jeong MH, Lim SY, Lee SR, Kim KH, Sohn IS, Park HW, Kim JH, Kim W, Ahn Y, Cho JG, Park JC, Kang JC. Elevated preprocedural high-sensitivity C-reactive protein levels are associated with neointimal hyperplasia and restenosis development after successful coronary artery stenting. Circ J. 2005. 69:1477–1483.
Article10. Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol. 1998. 31:224–230.
Article11. Grewe PH, Deneke T, Machraoui A, Barmeyer J, Muller KM. Acute and chronic tissue response to coronary stent implantation: pathologic findings in human specimen. J Am Coll Cardiol. 2000. 35:157–163.
Article12. Farb A, Sangiorgi G, Carter AJ, Walley VM, Edwards WD, Schwartz RS, Virmani R. Pathology of acute and chronic coronary stenting in humans. Circulation. 1999. 99:44–52.
Article13. Klugherz BD, Llanos G, Lieuallen W, Kopia GA, Papandreou G, Narayan P, Sasseen B, Adelman SJ, Falotico R, Wilensky RL. Twenty-eight-day efficacy and phamacokinetics of the sirolimus-eluting stent. Coron Artery Dis. 2002. 13:183–188.
Article14. Suzuki T, Kopia G, Hayashi S, Bailey LR, Llanos G, Wilensky R, Klugherz BD, Papandreou G, Narayan P, Leon MB, Yeung AC, Tio F, Tsao PS, Falotico R, Carter AJ. Stent-based delivery of sirolimus reduces neointimal formation in a porcine coronary model. Circulation. 2001. 104:1188–1193.
Article15. Carter AJ, Aggarwal M, Kopia GA, Tio F, Tsao PS, Kolata R, Yeung AC, Llanos G, Dooley J, Falotico R. Long-term effects of polymer-based, slow-release, sirolimus-eluting stents in a porcine coronary model. Cardiovasc Res. 2004. 63:617–624.
Article16. Heldman AW, Cheng L, Jenkins GM, Heller PF, Kim DW, Ware M Jr, Nater C, Hruban RH, Rezai B, Abella BS, Bunge KE, Kinsella JL, Sollott SJ, Lakatta EG, Brinker JA, Hunter WL, Froehlich JP. Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis. Circulation. 2001. 103:2289–2295.
Article17. Drachman DE, Edelman ER, Seifert P, Groothuis AR, Bornstein DA, Kamath KR, Palasis M, Yang D, Nott SH, Rogers C. Neointimal thickening after stent delivery of paclitaxel: change in composition and arrest of growth over six months. J Am Coll Cardiol. 2000. 36:2325–2332.
Article18. Farb A, Heller PF, Shroff S, Cheng L, Kolodgie FD, Carter AJ, Scott DS, Froehlich J, Virmani R. Pathological analysis of local delivery of paclitaxel via a polymer-coated stent. Circulation. 2001. 104:473–479.
Article19. EPIC (Evaluation of 7E3 in Preventing Ischemic Complications) Investigators. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. N Engl J Med. 1994. 330:956–961.20. EPILOG (Evaluation in PTCA to improve Long-Term outcome GP IIb/IIIa Blockade Study Group) Investigators. Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. N Engl J Med. 1997. 336:1689–1696.21. CAPTURE (C7E3 Fab AntiPlatelet Therapy in Unstable Refractory angina) Investigators. Randomized placebo-controlled trial of abciximab before and during coronary intervention in refractory unstable angina. Lancet. 1997. 349:1429–1435.22. Srivatsa SS, Fitzpatrick LA, Tsao PW, Reilly TM, Holmes DR Jr, Schwartz RS, Mousa SA. Selective alpha v beta 3 integrin blockade potently limits neointimal hyperplasia and lumen stenosis following deep coronary arterial stent injury: evidence for the functional importance of integrin alpha v beta 3 and osteopontin expression during neointima formation. Cardiovasc Res. 1997. 36:408–428.23. Shappell SB, Toman C, Anderson DC, Taylor AA, Entman ML, Smith CW. Mac-1 (CD11b/CD8) mediates adherence-dependent hydrogen peroxide production by human and canine neutrophils. J Immunol. 1990. 144:2702–2711.24. Simon DI, XU H, Ortlepp S, Rogers C, Rao NK. 7E3 monoclonal antibody directed against the platelet glycoprotein IIb/IIIa cross-reacts with the leukocyte integrin Mac-1 and blocks adhesion to fibrinogen and ICAM-1. Arterioscler Thromb Vasc Biol. 1997. 17:528–535.
Article25. Mickelson JK, Ali MN, Kleiman NS, Lakkis NM, Chow TW, Hughes BJ, Smith CW. Chimeric 7E3 Fab (ReoPro) decreases detectable CD IIb on neutrophils from patients undergoing coronary angioplasty. J Am Coll Cardiol. 1999. 33:97–106.26. Lefkovits J, Topol EJ. Platelet glycoprotein IIb/IIIa receptor antagonists in coronary artery disease. Eur Heart J. 1996. 17:9–18.
Article27. Kang KT, Jeong MH, Kim NH, Rhew JY, Lee SH, Park JC, Lee SU, Kim KH, Choi MJ, Ahn YK, Cho JG, Choi WJ, Cho DL, Park JT, Kang JC. The inhibitory effect of platelet glycoprotein IIb/IIIa receptor blocker-coated stent on porcine coronary stent restenosis. Korean J Med. 2001. 60:314–323.28. Park OY, Jeong MH, Kim JH, Kim W, Lee SH, Hong YJ, Kim JH, Park WS, Kim IS, Choi MJ, Ahn YK, Park JT, Cho JG, Park JC, Cho DL, Kang JC. The inhibitory effects of platelet glycoprotein IIb/IIIa receptor blocker-coated stent on neointima formation and inflammatory response in porcine coronary stent restenosis. Korean Circ J. 2003. 33:439–445.
Article29. Hong YJ, Jeong MH, Kim W, Lim SY, Hong SN, Lee SH, Kim KH, Yun KH, Kang DG, Lee YS, Park HW, Kim JH, Ahn Y, Cho JG, Park JT, Park CS, Park JC, Kang JC. The effects of abciximab (Reo-Pro(r))-coated stents on extracellular matrix synthesis and apoptosis. Korean Circ J. 2005. 35:290–301.30. Schwartz RS, Huber KC, Murphy JG, Edwards WD, Camrud AR, Vlietstra RE, Holmes DR. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992. 19:267–274.
Article31. Salame MY, Verheye S, Crocker IR, Chronos NA, Robinson KA, King SB 3rd. Intracoronary radiation therapy. Eur Heart J. 2001. 22:629–647.
Article32. Faxon DP. Systemic drug therapy for restenosis: "deja vu all over again". Circulation. 2002. 106:2296–2298.33. Versaci F, Gaspardone A, Tomai F, Ribichini F, Russo P, Proietti I, Ghini AS, Ferrero V, Chiariello L, Gioffre PA, Romeo F, Crea F. Immunosuppressive Therapy for the Prevention of Restenosis after Coronary Artery Stent Implantation Study. Immunosuppressive Therapy for the Prevention of Restenosis after Coronary Artery Stent Implantation (IMPRESS Study). J Am Coll Cardiol. 2002. 40:1935–1942.
Article34. Lincoff AM, Topol EJ, Ellis SG. Local drug delivery for the prevention of restenosis. Fact, fancy, and future. Circulation. 1994. 90:2070–2084.
Article35. Wohrle J, Al-Khayer E, Grotzinger U, Schindler C, Kochs M, Hombach V, Hoher M. Comparison of the heparin coated vs the uncoated Jostent--no influence on restenosis or clinical outcome. Eur Heart J. 2001. 22:1808–1816.36. Lemos PA, Serruys PW, Sousa JE. Drug-eluting stents: cost versus clinical benefit. Circulation. 2003. 107:3003–3007.37. O'Neill WW, Leon MB. Drug-eluting stents: costs versus clinical benefit. Circulation. 2003. 107:3008–3011.38. Kereiakes DJ. Hippocrates revisited: the evidence for drug-eluting stents. Circulation. 2003. 107:3012–3014.39. Hong YJ, Jeong MH, Kim W, Lim SY, Lee SH, Hong SN, Kim JH, Ahn YK, Cho JG, Park JC, Cho DL, Kim H, Kang JC. Effect of abciximab-coated stent on in-stent intimal hyperplasia in human coronary arteries. Am J Cardiol. 2004. 94:1050–1054.
Article40. Kim W, Jeong MH, Kim KH, Sohn IS, Hong YJ, Park HW, Kim JH, Ahn YK, Cho JG, Park JC, Cho DL, Kang JC. The clinical results of a platelet glycoprotein IIb/IIIa receptor blocker (abciximab: Reo-Pro)-coated stent in acute myocardial infarction. J Am Coll Cardiol. 2006. 47:933–938.
Article41. Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med. 1992. 326:242–250.42. Willerson JT, Golino P, Eidt J, Campbell WB, Buja LM. Specific platelet mediators and unstable coronary artery lesions. Experimental evidence and potential clinical implications. Circulation. 1989. 80:198–205.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of a Dual Drug-Coated Stent With Abciximab and Alpha-Lipoic Acid in a Porcine Coronary Restenosis Model
- The preventive effects of the heparin-coated coronary stent in a porcine coronary stent restenosis model
- The preventive effects of the heparin-coated coronary stent in a porcine coronary stent restenosis model
- The Effects of the Heparin-Coated Maximum Arterial Re-Creation (MAC) Stent on Porcine Coronary Stent Restenosis
- The Inhibitory Effects of Platelet Glycoprotein IIb/IIIa Receptor Blocker-Coated Stent on Neointima Formation and Inflammatory Response in Porcine Coronary Stent Restenosis