Korean Circ J.
2011 Sep;41(9):518-527. 10.4070/kcj.2011.41.9.518.
Changes of Pulmonary Pathology and Gene Expressions After Simvastatin Treatment in the Monocrotaline-Induced Pulmonary Hypertension Rat Model
- Affiliations
-
- 1Department of Pediatrics, School of Medicine, Ewha Womans University, Seoul, Korea. ymhong@ewha.ac.kr
- 2Department of Thoracic and Cardiovascular Surgery, School of Medicine, Ewha Womans University, Seoul, Korea.
- 3Department of Pathology, School of Medicine, Ewha Womans University, Seoul, Korea.
- KMID: 2094104
- DOI: http://doi.org/10.4070/kcj.2011.41.9.518
Abstract
- BACKGROUND AND OBJECTIVES
Simvastatin's properties are suggestive of a potential pathophysiologic role in pulmonary hypertension. The objectives of this study were to investigate changes of pulmonary pathology and gene expressions, including endothelin (ET)-1, endothelin receptor A (ERA), inducible nitric oxide synthase (NOS2), endothelial nitric oxide synthase (NOS3), matrix metalloproteinase (MMP) 2, tissue inhibitor of matrix metalloproteinases (TIMP) and caspase 3, and to evaluate the effect of simvastatin on monocrotaline (M)-induced pulmonary hypertension.
MATERIALS AND METHODS
Six week old male Sprague-Dawley rats were treated, as follows: control group, subcutaneous (sc) injection of saline; M group, sc injection of M (60 mg/kg); and simvastatin group, sc injection of M (60 mg/kg) plus 10 mg/kg/day simvastatin orally.
RESULTS
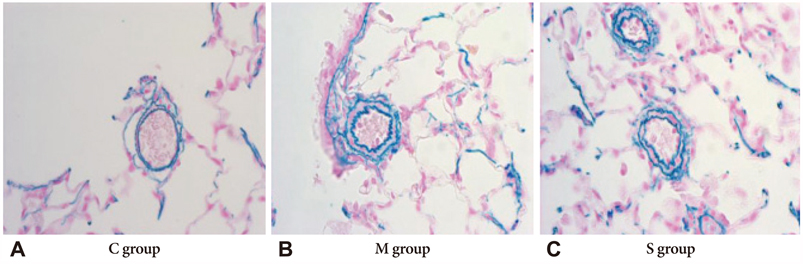
On day 28, right ventricular hypertrophy (RVH) significantly decreased in the simvastatin group compared to the M group. Similarly, right ventricular pressure significantly decreased in the simvastatin group on day 28. From day 7, the ratio of medial thickening of the pulmonary artery was significantly increased in the M group, but there was no significant change in the simvastatin group. The number of muscular pulmonary arterioles was significantly reduced in the simvastatin group. On day 5, gene expressions of ET-1, ERA, NOS2, NOS3, MMP and TIMP significantly decreased in the simvastatin group.
CONCLUSION
Administration of simvastatin exerted weak inhibitory effects on RVH and on the number of muscular pulmonary arterioles, during the development of M-induced pulmonary hypertension in rats. Simvastatin decreased gene expressions on day 5.
MeSH Terms
-
Animals
Arterioles
Caspase 3
Endothelins
Gene Expression
Humans
Hypertension, Pulmonary
Hypertrophy, Right Ventricular
Male
Matrix Metalloproteinases
Monocrotaline
Nitric Oxide Synthase Type II
Nitric Oxide Synthase Type III
Pulmonary Artery
Rats
Rats, Sprague-Dawley
Receptors, Endothelin
Simvastatin
Ventricular Pressure
Caspase 3
Endothelins
Matrix Metalloproteinases
Monocrotaline
Nitric Oxide Synthase Type II
Nitric Oxide Synthase Type III
Receptors, Endothelin
Simvastatin
Figure
Cited by 1 articles
-
Microarray Analysis in Pulmonary Hypertensive Rat Heart after Simvastatin Treatment
Yi Kyung Kim, Kwan Chang Kim, Young Mi Hong
Ewha Med J. 2018;41(3):53-62. doi: 10.12771/emj.2018.41.3.53.
Reference
-
1. Humbert M, Morrell NW, Archer SL, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004. 43:12 Suppl S. 13S–24S.2. Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004. 109:159–165.3. Meininger GA, Davis MJ. Cellular mechanisms involved in the vascular myogenic response. Am J Physiol. 1992. 263:H647–H659.4. Keck EW. Pulmonary hypertension and pulmonary vascular disease in congenital heart defects. Z Kardiol. 1989. 78:Suppl 7. 65–73.5. Yamaki S, Endo M, Takahashi T. Different grades of medial hypertrophy and intimal changes in small pulmonary arteries among various types of congenital heart disease with pulmonary hypertension. Tohoku J Exp Med. 1997. 182:83–91.6. Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988. 333:664–666.7. Todd L, Mullen M, Olley PM, Rabinovitch M. Pulmonary toxicity of monocrotaline differs at critical periods of lung development. Pediatr Res. 1985. 19:731–737.8. Girgis RE, Li D, Zhan X, et al. Attenuation of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Heart Circ Physiol. 2003. 285:H938–H945.9. Nishimura T, Faul JL, Berry GJ, et al. Simvastatin attenuates smooth muscle neointimal proliferation and pulmonary hypertension in rats. Am J Respir Crit Care Med. 2002. 166:1403–1408.10. Nishimura T, Vaszar LT, Faul JL, et al. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation. 2003. 108:1640–1645.11. McMurtry MS, Bonnet S, Michelakis ED, Bonnet S, Haromy A, Archer SL. Statin therapy, alone or with rapamycin, does not reverse monocrotaline pulmonary arterial hypertension: the rapamycin-atorvastatin-simvastatin study. Am J Physiol Lung Cell Mol Physiol. 2007. 293:L933–L940.12. Kim MY, Kim YK, Jung YW, Kim WT, Kwon TH, Lee DS. The protective effect of simvastatin on monocrotaline-induced pulmonary hypertension in rats. Korean Circ J. 2008. 38:313–319.13. Taraseviciene-Stewart L, Scerbavicius R, Choe KH, et al. Simvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006. 291:L668–L676.14. Lim KA, Shim JY, Cho SH, Kim KW, Han JJ, Hong YM. Effect of endothelin receptor blokade on monocrotaline-induced pulmonary hypertension in rats. Korean J Pediatr. 2009. 52:689–695.15. Lim KA, Kim KC, Cho MS, Lee BE, Kim HS, Hong YM. Gene expression of endothelin-1 and endothelin receptor A on monocrotaline-induced pulmonary hypertension in rats after bosentan treatment. Korean Circ J. 2010. 40:459–464.16. Koo HS, Kim KC, Hong YM. Gene expression of nitric oxide synthase and matrix metalloproteinase-2 in monocrotaline-induced pulmonary hypertension in rats after bosentan treatment. Korean Circ J. 2011. 41:83–90.17. Miyauchi T, Yorikane R, Sakai S, et al. Contribution of endogenous endothelin-1 to the progression of cardiopulmonary alterations in rats with monocrotalin-induced pulmonary hypertension. Circ Res. 1993. 73:887–897.18. Itoh T, Nagaya N, Fujii T, et al. A combination of oral sildenafil and beraprost ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med. 2004. 169:34–38.19. Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation. 2000. 101:207–213.20. Beckman JS, Koppenol WH. Nitric oxide, superoxide and peroxynitrite: the good, the bad, and the ugly. Am J Physiol. 1996. 271:C1424–C1437.21. Hampl V, Herget J. Role of nitric oxide in the pathogenesis of chronic pulmonary hypertension. Physiol Rev. 2000. 80:1337–1372.22. Miller AA, Hislop AA, Vallance PJ, Haworth SG. Deletion of the eNOS gene has a greater impact on the pulmonary circulation of male than female mice. Am J Physiol Lung Cell Mol Physiol. 2005. 289:L299–L306.23. Li M, Li Z, Sun X. Statins suppress MMP2 secretion via inactivation of RhoA/ROCK pathway in pulmonary vascular smooth muscle cells. Eur J Pharmacol. 2008. 591:219–223.24. Girgis RE, Mozammel S, Champion HC, et al. Regression of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Lung Cell Mol Physiol. 2007. 292:L1105–L1110.25. Kim YC, Song SB, Lee MH, et al. Simvastatin induces caspase-independent apoptosis in LPS-activated RAW264.7 macrophage cells. Biochem Biophys Res Commun. 2006. 339:1007–1014.26. Kaneta S, Satoh K, Kano S, Kanda M, Ichihara K. All hydrophobic HMG-CoA reductase inhibitors induce apoptotic death in rat pulmonary vein endothelial cells. Atherosclerosis. 2003. 170:237–243.27. Kubota T, Fujisaki K, Itoh Y, Yano T, Sendo T, Oishi R. Apoptotic injury in cultured human hepatocytes induced by HMG-CoA reductase inhibitors. Biochem Pharmacol. 2004. 67:2175–2186.28. Wilkins MR, Ali O, Bradlow W, et al. Simvastatin as a treatment for pulmonary hypertension trial. Am J Respir Crit Care Med. 2010. 181:1106–1113.29. Liu B, Wang XQ, Yu L, Zhou TF, Wang XM, Liu HM. Simvastatin restores down-regulated GATA-6 expression in pulmonary hypertensive rats. Exp Lung Res. 2009. 35:411–426.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Microarray Analysis in Pulmonary Hypertensive Rat Heart after Simvastatin Treatment
- An inhibitory effect of tumor necrosis factor-alpha antagonist to gene expression in monocrotaline-induced pulmonary hypertensive rats model
- The Protective Effect of Simvastatin on Monocrotaline-Induced Pulmonary Hypertension in Rats
- Simvastatin, Sildenafil and Their Combination in Monocrotaline Induced Pulmonary Arterial Hypertension
- Effects of Bosentan Treatment on Angiotensin Converting Enzyme in Monocrotaline Induced Pulmonary Hypertension Rats