Rediscovery of Nefopam for the Treatment of Neuropathic Pain
- Affiliations
-
- 1Department of Anesthesia and Pain Medicine, School of Medicine, Pusan National University, Yangsan, Korea.
- 2Department of Pain Medicine, Division of Anesthesiology and Critical Care, The University of Texas MD Anderson Cancer Center, Houston, TX, USA. SAbdi@mdanderson.org
- KMID: 2074055
- DOI: http://doi.org/10.3344/kjp.2014.27.2.103
Abstract
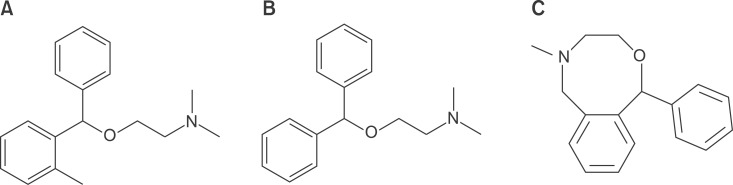
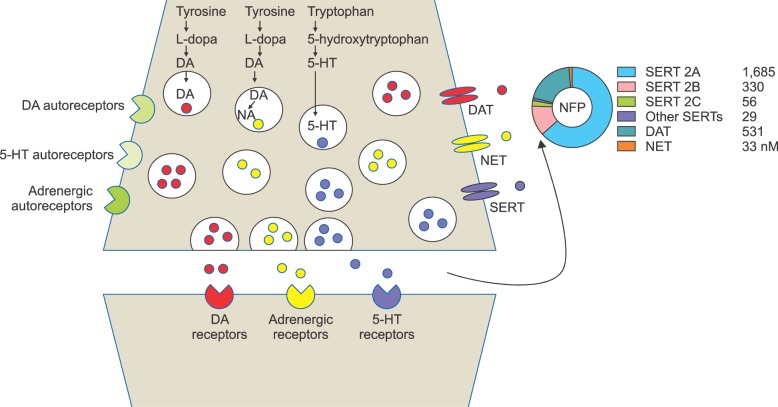
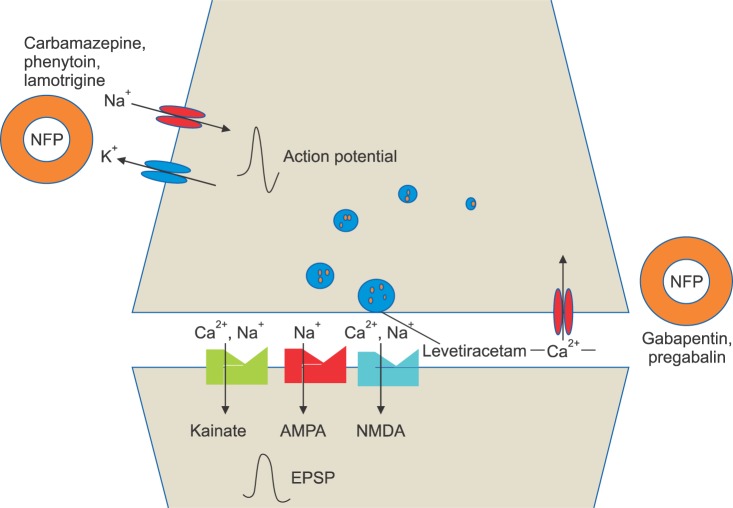
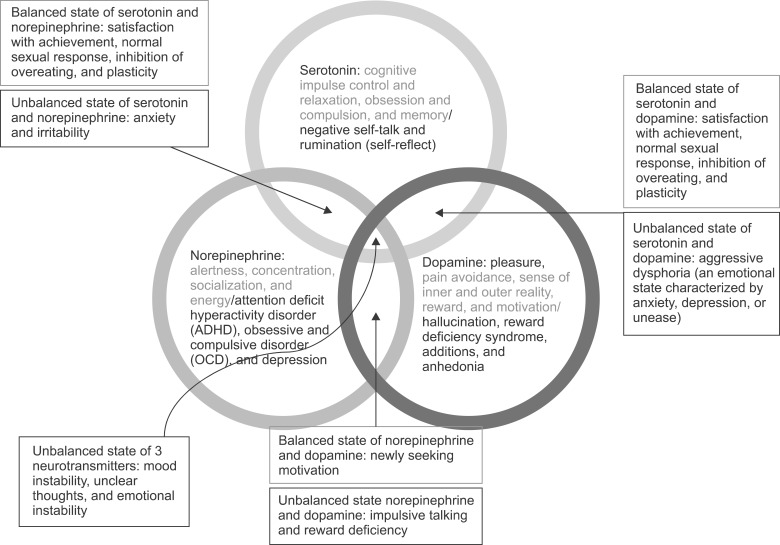
- Nefopam (NFP) is a non-opioid, non-steroidal, centrally acting analgesic drug that is derivative of the non-sedative benzoxazocine, developed and known in 1960s as fenazocine. Although the mechanisms of analgesic action of NFP are not well understood, they are similar to those of triple neurotransmitter (serotonin, norepinephrine, and dopamine) reuptake inhibitors and anticonvulsants. It has been used mainly as an analgesic drug for nociceptive pain, as well as a treatment for the prevention of postoperative shivering and hiccups. Based on NFP's mechanisms of analgesic action, it is more suitable for the treatment of neuropathic pain. Intravenous administration of NFP should be given in single doses of 20 mg slowly over 15-20 min or with continuous infusion of 60-120 mg/d to minimize adverse effects, such as nausea, cold sweating, dizziness, tachycardia, or drowsiness. The usual dose of oral administration is three to six times per day totaling 90-180 mg. The ceiling effect of its analgesia is uncertain depending on the mechanism of pain relief. In conclusion, the recently discovered dual analgesic mechanisms of action, namely, a) descending pain modulation by triple neurotransmitter reuptake inhibition similar to antidepressants, and b) inhibition of long-term potentiation mediated by NMDA from the inhibition of calcium influx like gabapentinoid anticonvulsants or blockade of voltage-sensitive sodium channels like carbamazepine, enable NFP to be used as a therapeutic agent to treat neuropathic pain.
Keyword
MeSH Terms
-
Administration, Intravenous
Administration, Oral
Analgesia
Analgesics, Non-Narcotic
Anticonvulsants
Antidepressive Agents
Calcium
Carbamazepine
Dizziness
Drug-Related Side Effects and Adverse Reactions
Hiccup
Long-Term Potentiation
Molecular Mechanisms of Pharmacological Action
N-Methylaspartate
Nausea
Nefopam*
Neuralgia*
Neurotransmitter Agents
Nociceptive Pain
Norepinephrine
Shivering
Sleep Stages
Sodium Channels
Sweat
Sweating
Tachycardia
Analgesics, Non-Narcotic
Anticonvulsants
Antidepressive Agents
Calcium
Carbamazepine
Molecular Mechanisms of Pharmacological Action
N-Methylaspartate
Nefopam
Neurotransmitter Agents
Norepinephrine
Sodium Channels
Figure
Cited by 14 articles
-
Effects on postoperative nausea and vomiting of nefopam versus fentanyl following bimaxillary orthognathic surgery: a prospective double-blind randomized controlled trial
Eunhye Choi, Myong-Hwan Karm, Eunsun So, Yoon Ji Choi, Sookyung Park, Yul Oh, Hye Joo Yun, Hyun Jeong Kim, Kwang-Suk Seo
J Dent Anesth Pain Med. 2019;19(1):55-66. doi: 10.17245/jdapm.2019.19.1.55.Comparison of effect of electroacupuncture and nefopam for prevention of postanesthetic shivering in patients undergoing urologic operation under spinal anesthesia
Jun-Ho Hong, Su-Jin Kim, Min-Sub Hwang
Korean J Anesthesiol. 2016;69(6):579-586. doi: 10.4097/kjae.2016.69.6.579.Emergence agitation: current knowledge and unresolved questions
Seok-Jin Lee, Tae-Yun Sung
Korean J Anesthesiol. 2020;73(6):471-485. doi: 10.4097/kja.20097.For the peace of postanesthesia care unit
Sung Mi Hwang
Korean J Anesthesiol. 2018;71(3):173-174. doi: 10.4097/kja.d.18.00113.Effects of nefopam on catheter-related bladder discomfort in patients undergoing ureteroscopic litholapaxy
Yong Woo Cheon, Seon Hwan Kim, Jin Hyub Paek, Jin A Kim, Yong Kyung Lee, Jin Hye Min, Hyung Rae Cho
Korean J Anesthesiol. 2018;71(3):201-206. doi: 10.4097/kja.d.18.27113.All about pain pharmacology: what pain physicians should know
Kyung-Hoon Kim, Hyo-Jung Seo, Salahadin Abdi, Billy Huh
Korean J Pain. 2020;33(2):108-120. doi: 10.3344/kjp.2020.33.2.108.The Potential Role of Intrathecal Nefopam in the Management of Neuropathic Pain
Mohamed Amin Ghobadifar, Navid Kalani
Korean J Pain. 2014;27(3):301-302. doi: 10.3344/kjp.2014.27.3.301.The Effect of Nefopam on Postoperative Fentanyl Consumption: A Randomized, Double-blind Study
Jee Youn Moon, Sang Sik Choi, Shin Young Lee, Mi Kyung Lee, Jung Eun Kim, Ji Eun Lee, So Hyun Lee
Korean J Pain. 2016;29(2):110-118. doi: 10.3344/kjp.2016.29.2.110.The Role of Spinal Dopaminergic Transmission in the Analgesic Effect of Nefopam on Rat Inflammatory Pain
Do Yun Kim, Joo Wung Chae, Chang Hun Lim, Bong Ha Heo, Keun Suk Park, Hyung Gon Lee, Jeong Il Choi, Myung Ha Yoon, Woong Mo Kim
Korean J Pain. 2016;29(3):164-171. doi: 10.3344/kjp.2016.29.3.164.Tapentadol: Can It Kill Two Birds with One Stone without Breaking Windows?
Eun Jung Chang, Eun Ji Choi, Kyung Hoon Kim
Korean J Pain. 2016;29(3):153-157. doi: 10.3344/kjp.2016.29.3.153.Nefopam Reduces Dysesthesia after Percutaneous Endoscopic Lumbar Discectomy
Young Min Ok, Ji Hyun Cheon, Eun Ji Choi, Eun Jung Chang, Ho Myung Lee, Kyung Hoon Kim
Korean J Pain. 2016;29(1):40-47. doi: 10.3344/kjp.2016.29.1.40.Use of Nefopam in Perioperative Pain Management; Keeping Nefopam in between
Jeong Il Choi
Korean J Pain. 2016;29(2):71-72. doi: 10.3344/kjp.2016.29.2.71.Earlier treatment improves the chances of complete relief from postherpetic neuralgia
Dong Hee Kang, Su Young Kim, Hyuck Goo Kim, Jung Hyun Park, Tae Kyun Kim, Kyung Hoon Kim
Korean J Pain. 2017;30(3):214-219. doi: 10.3344/kjp.2017.30.3.214.Status Epilepticus Caused by Nefopam
Yong-sook Park, Young-baeg Kim, Jeong-min Kim
J Korean Neurosurg Soc. 2014;56(5):448-450. doi: 10.3340/jkns.2014.56.5.448.
Reference
-
1. Gregori-Puigjané E, Setola V, Hert J, Crews BA, Irwin JJ, Lounkine E, et al. Identifying mechanism-of-action targets for drugs and probes. Proc Natl Acad Sci U S A. 2012; 109:11178–11183. PMID: 22711801.
Article2. Moore RA, Derry S, McQuay HJ, Wiffen PJ. Single dose oral analgesics for acute postoperative pain in adults. Cochrane Database Syst Rev. 2011; (9):CD008659. PMID: 21901726.
Article3. Alfonsi P, Adam F, Passard A, Guignard B, Sessler DI, Chauvin M. Nefopam, a nonsedative benzoxazocine analgesic, selectively reduces the shivering threshold in unanesthetized subjects. Anesthesiology. 2004; 100:37–43. PMID: 14695722.
Article4. Heel RC, Brogden RN, Pakes GE, Speight TM, Avery GS. Nefopam: a review of its pharmacological properties and therapeutic efficacy. Drugs. 1980; 19:249–267. PMID: 6991238.5. Podranski T, Bouillon TW, Riva T, Kurz AM, Oehmke MJ. Compartmental pharmacokinetics of nefopam during mild hypothermia. Br J Anaesth. 2012; 108:784–791. PMID: 22331396.
Article6. Bassett JR, Cairncross KD, Hacket NB, Story M. Studies on the peripheral pharmacology of fenazoxine, a potential antidepressant drug. Br J Pharmacol. 1969; 37:69–78. PMID: 5343358.
Article7. Tobin WE, Gold RH. Nefopam hydrochloride: a novel muscle relaxant. J Clin Pharmacol New Drugs. 1972; 12:230–238. PMID: 4555883.
Article8. Bolt AG, Graham G, Wilson P. Stereoselective demethylation of the enantiomers of nefopam, an experimental antidepressant and skeletal muscle relaxant. Xenobiotica. 1974; 4:355–363. PMID: 4842015.
Article9. Koe BK. Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. J Pharmacol Exp Ther. 1976; 199:649–661. PMID: 994022.10. Cohen A. Nefopam hydrochloride for pain relief. Curr Ther Res Clin Exp. 1974; 16:184–193. PMID: 4206725.11. Klotz AL. Long-term safety of Nefopam hydrochloride (Acupan), a new analgesic formulation. Curr Ther Res Clin Exp. 1974; 16:602–608. PMID: 4211140.12. Workmon FC, Winter L Jr. A clinical evaluation of nefopam hydrochloride (Acupan): a new analgesic. Curr Ther Res Clin Exp. 1974; 16:609–616. PMID: 4211141.13. Kolodny AL, Winter L Jr. Further clinical evaluations of nefopam hydrochloride, a new analgesic. Curr Ther Res Clin Exp. 1975; 17:519–524. PMID: 808373.14. Kakkar M, Derry S, Moore RA, McQuay HJ. Single dose oral nefopam for acute postoperative pain in adults. Cochrane Database Syst Rev. 2009; (3):CD007442. PMID: 19588431.
Article15. Izzo V, Mariconti P, Tiengo M. Action and effectiveness of nefopam chloride in the control of postoperative shivering. Minerva Anestesiol. 1991; 57:760–762. PMID: 1798568.16. Kim YA, Kweon TD, Kim M, Lee HI, Lee YJ, Lee KY. Comparison of meperidine and nefopam for prevention of shivering during spinal anesthesia. Korean J Anesthesiol. 2013; 64:229–233. PMID: 23560188.
Article17. Park SM, Mangat HS, Berger K, Rosengart AJ. Efficacy spectrum of antishivering medications: meta-analysis of randomized controlled trials. Crit Care Med. 2012; 40:3070–3082. PMID: 22890247.18. Bilotta F, Rosa G. Nefopam for severe hiccups. N Engl J Med. 2000; 343:1973–1974. PMID: 11186682.
Article19. Bilotta F, Pietropaoli P, Rosa G. Nefopam for refractory postoperative hiccups. Anesth Analg. 2001; 93:1358–1360. PMID: 11682430.
Article20. Pajot S, Geeraerts T, Leblanc PE, Duranteau J, Benhamou D. Hiccup during weaning from mechanical ventilation: the use of nefopam. Br J Anaesth. 2007; 99:748–749. PMID: 17933805.
Article21. Barrot M. Tests and models of nociception and pain in rodents. Neuroscience. 2012; 211:39–50. PMID: 22244975.
Article22. Jaggi AS, Jain V, Singh N. Animal models of neuropathic pain. Fundam Clin Pharmacol. 2011; 25:1–28. PMID: 20030738.
Article23. Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987; 30:103–114. PMID: 3614974.
Article24. Cho SY, Park AR, Yoon MH, Lee HG, Kim WM, Choi JI. Antinociceptive effect of intrathecal nefopam and interaction with morphine in formalin-induced pain of rats. Korean J Pain. 2013; 26:14–20. PMID: 23342202.
Article25. Girard P, Pansart Y, Coppe MC, Gillardin JM. Nefopam reduces thermal hypersensitivity in acute and postoperative pain models in the rat. Pharmacol Res. 2001; 44:541–545. PMID: 11735363.
Article26. Buritova J, Besson JM. Effects of nefopam on the spinal nociceptive processes: a c-Fos protein study in the rat. Eur J Pharmacol. 2002; 441:67–74. PMID: 12007921.
Article27. Laboureyras E, Chateauraynaud J, Richebé P, Simonnet G. Long-term pain vulnerability after surgery in rats: prevention by nefopam, an analgesic with antihyperalgesic properties. Anesth Analg. 2009; 109:623–631. PMID: 19608840.
Article28. Evans MS, Lysakowski C, Tramèr MR. Nefopam for the prevention of postoperative pain: quantitative systematic review. Br J Anaesth. 2008; 101:610–617. PMID: 18796441.
Article29. Tigerstedt I, Tammisto T, Leander P. Comparison of the analgesic dose-effect relationships of nefopam and oxycodone in postoperative pain. Acta Anaesthesiol Scand. 1979; 23:555–560. PMID: 397711.
Article30. Dordoni PL, Della Ventura M, Stefanelli A, Iannace E, Paparella P, Rocca B, et al. Effect of ketorolac, ketoprofen and nefopam on platelet function. Anaesthesia. 1994; 49:1046–1049. PMID: 7864317.
Article31. Gasser JC, Bellville JW. Respiratory effects of nefopam. Clin Pharmacol Ther. 1975; 18:175–179. PMID: 1097153.
Article32. Guindon J, Walczak JS, Beaulieu P. Recent advances in the pharmacological management of pain. Drugs. 2007; 67:2121–2133. PMID: 17927280.
Article33. Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003; 4:13–25. PMID: 12511858.
Article34. Max MB, Gilron IH. Antidepressants, muscle relaxants, and N-methyl-D-aspartate receptor antagonists. In : Loeser JD, Bonica JJ, editors. Bonica's management of pain. 3rd ed. Philadelphia (PA): Lippincott Williams & Wilkins;2001. p. 1710–1726.35. Novelli A, Díaz-Trelles R, Groppetti A, Fernández-Sánchez MT. Nefopam inhibits calcium influx, cGMP formation, and NMDA receptor-dependent neurotoxicity following activation of voltage sensitive calcium channels. Amino Acids. 2005; 28:183–191. PMID: 15714253.
Article36. Verleye M, André N, Heulard I, Gillardin JM. Nefopam blocks voltage-sensitive sodium channels and modulates glutamatergic transmission in rodents. Brain Res. 2004; 1013:249–255. PMID: 15193535.
Article37. Biella GE, Groppetti A, Novelli A, Fernández-Sánchez MT, Manfredi B, Sotgiu ML. Neuronal sensitization and its behavioral correlates in a rat model of neuropathy are prevented by a cyclic analog of orphenadrine. J Neurotrauma. 2003; 20:593–601. PMID: 12906743.
Article38. Novelli A, Groppetti A, Rossoni G, Manfredi B, Ferrero-Gutiérrez A, Pérez-Gómez A, et al. Nefopam is more potent than carbamazepine for neuroprotection against veratridine in vitro and has anticonvulsant properties against both electrical and chemical stimulation. Amino Acids. 2007; 32:323–332. PMID: 17021653.
Article39. Czuczwar M, Czuczwar K, Cięszczyk J, Kiś J, Saran T, Łuszczki JJ, et al. Nefopam enhances the protective activity of antiepileptics against maximal electroshock-induced convulsions in mice. Pharmacol Rep. 2011; 63:690–696. PMID: 21857079.
Article40. Löscher W, Schmidt D. New Horizons in the development of antiepileptic drugs: innovative strategies. Epilepsy Res. 2006; 69:183–272. PMID: 16835945.
Article41. Hoebel BG, Hernandez L, Schwartz DH, Mark GP, Hunter GA. Microdialysis studies of brain norepinephrine, serotonin, and dopamine release during ingestive behavior. Theoretical and clinical implications. Ann N Y Acad Sci. 1989; 575:171–191. PMID: 2699187.
Article42. Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev. 1994; 46:157–203. PMID: 7938165.43. Fink KB, Göthert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev. 2007; 59:360–417. PMID: 18160701.
Article44. Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999; 38:1083–1152. PMID: 10462127.
Article45. Bannister K, Bee LA, Dickenson AH. Preclinical and early clinical investigations related to monoaminergic pain modulation. Neurotherapeutics. 2009; 6:703–712. PMID: 19789074.
Article46. Cotecchia S, Stanasila L, Diviani D. Protein-protein interactions at the adrenergic receptors. Curr Drug Targets. 2012; 13:15–27. PMID: 21777184.
Article47. Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol. 2004; 61:641–644. PMID: 15148138.
Article48. Benzon HT. The neuropathic pain scales. Reg Anesth Pain Med. 2005; 30:417–421. PMID: 16135344.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Use of Nefopam in Perioperative Pain Management; Keeping Nefopam in between
- The Potential Role of Intrathecal Nefopam in the Management of Neuropathic Pain
- Intravenous Nefopam Reduces Postherpetic Neuralgia during the Titration of Oral Medications
- Enhancement of Antinociception by Co-administrations of Nefopam, Morphine, and Nimesulide in a Rat Model of Neuropathic Pain
- Effects of Nefopam on Streptozotocin-Induced Diabetic Neuropathic Pain in Rats