Enhancement of Antinociception by Co-administrations of Nefopam, Morphine, and Nimesulide in a Rat Model of Neuropathic Pain
- Affiliations
-
- 1Department of Pharmacology & Neuroscience Research Center, Shahid Beheshti Medical University, Tehran, Iran. tzanjani@yahoo.com
- KMID: 2278109
- DOI: http://doi.org/10.3344/kjp.2012.25.1.7
Abstract
- BACKGROUND
Neuropathic pain is a chronic pain due to disorder in the peripheral or central nervous system with different pathophysiological mechanisms. Current treatments are not effective. Analgesic drugs combined can reduce pain intensity and side effects. Here, we studied the analgesic effect of nimesulide, nefopam, and morphine with different mechanisms of action alone and in combination with other drugs in chronic constriction injury (CCI) model of neuropathic pain.
METHODS
Male Wistar rats (n = 8) weighing 150-200 g were divided into 3 different groups: 1- Saline-treated CCI group, 2- Saline-treated sham group, and 3- Drug-treated CCI groups. Nimesulide (1.25, 2.5, and 5 mg/kg), nefopam (10, 20, and 30 mg/kg), and morphine (1, 3, and 5 mg/kg) were injected 30 minutes before surgery and continued daily to day 14 post-ligation. In the combination strategy, a nonanalgesic dose of drugs was used in combination such as nefopam + morphine, nefopam + nimesulide, and nimesulide + morphine. Von Frey filaments for mechanical allodynia and acetone test for cold allodynia were, respectively, used as pain behavioral tests. Experiments were performed on day 0 (before surgery) and days 1, 3, 5, 7, 10, and 14 post injury.
RESULTS
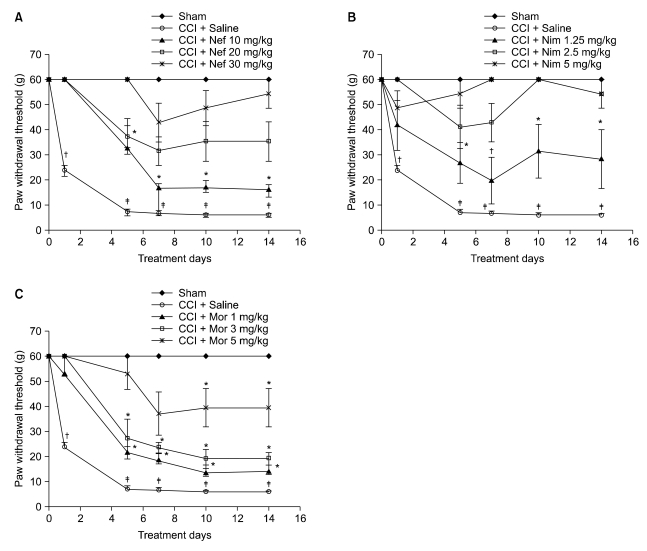
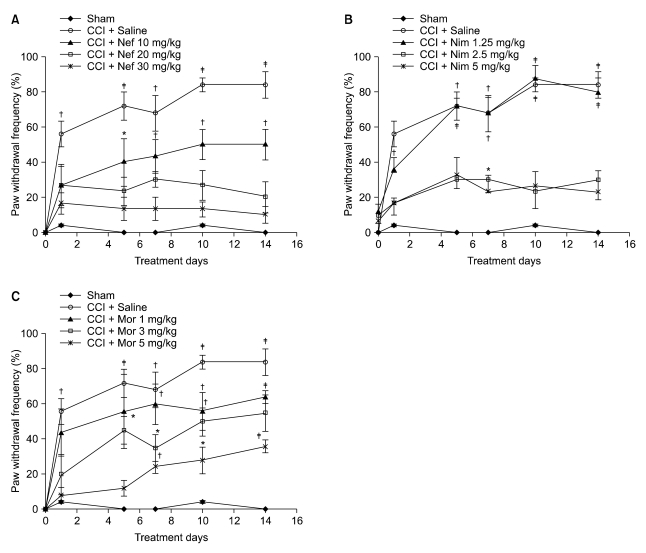
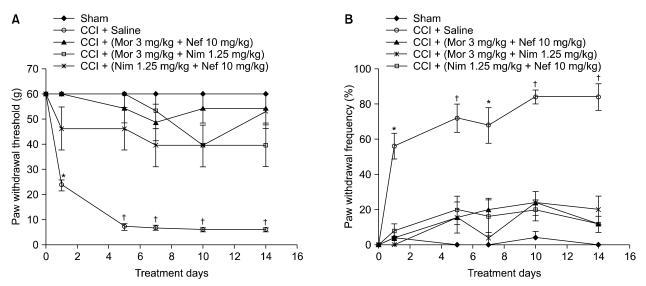
Nefopam (30 mg/kg) and nimesulide (5 mg/kg) blocked mechanical and thermal allodynia; the analgesic effects of morphine (5 mg/kg) lasted for 7 days. Allodynia was completely inhibited in combination with nonanalgesic doses of nefopam (10 mg/kg), nimesulide (1.25 mg/kg), and morphine (3 mg/kg).
CONCLUSIONS
It seems that analgesic drugs used in combination, could effectively reduce pain behavior with reduced adverse effects.
Keyword
MeSH Terms
Figure
Cited by 7 articles
-
Emerging Anti Carcinogenic Applications of Nimesulide: Therapeutic Benefits Beyond Its Primary Role in Pain Management
Shailendra Kapoor
Korean J Pain. 2012;25(3):198-199. doi: 10.3344/kjp.2012.25.3.198.Antinociceptive Effect of Intrathecal Nefopam and Interaction with Morphine in Formalin-Induced Pain of Rats
Soo Young Cho, A Reum Park, Myung Ha Yoon, Hyung Gon Lee, Woong Mo Kim, Jeong Il Choi
Korean J Pain. 2013;26(1):14-20. doi: 10.3344/kjp.2013.26.1.14.The Analgesic Effect of Nefopam with Fentanyl at the End of Laparoscopic Cholecystectomy
Ju Hwan Lee, Jae Hong Kim, Yong Kwan Cheong
Korean J Pain. 2013;26(4):361-367. doi: 10.3344/kjp.2013.26.4.361.Medications in Treatment of Postherpetic Neuralgia
Sang Wook Shin
Korean J Pain. 2014;27(1):1-2. doi: 10.3344/kjp.2014.27.1.1.The Potential Role of Intrathecal Nefopam in the Management of Neuropathic Pain
Mohamed Amin Ghobadifar, Navid Kalani
Korean J Pain. 2014;27(3):301-302. doi: 10.3344/kjp.2014.27.3.301.Effects of Nefopam on Streptozotocin-Induced Diabetic Neuropathic Pain in Rats
Jae Sik Nam, Yu Seon Cheong, Myong Hwan Karm, Ho Soo Ahn, Ji Hoon Sim, Jin Sun Kim, Seong Soo Choi, Jeong Gil Leem
Korean J Pain. 2014;27(4):326-333. doi: 10.3344/kjp.2014.27.4.326.Use of Nefopam in Perioperative Pain Management; Keeping Nefopam in between
Jeong Il Choi
Korean J Pain. 2016;29(2):71-72. doi: 10.3344/kjp.2016.29.2.71.
Reference
-
1. Blackburn-Munro G. Pain-like behaviours in animals - how human are they? Trends Pharmacol Sci. 2004; 25:299–305. PMID: 15165744.
Article2. Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002; 18:350–354. PMID: 12441828.
Article3. Moulin DE, Clark AJ, Gilron I, Ware MA, Watson CP, Sessle BJ, et al. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007; 12:13–21. PMID: 17372630.
Article4. Suzuki R, Dickenson AH. Differential pharmacological modulation of the spontaneous stimulus-independent activity in the rat spinal cord following peripheral nerve injury. Exp Neurol. 2006; 198:72–80. PMID: 16336968.
Article5. Arnér S, Meyerson BA. Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain. 1988; 33:11–23. PMID: 2454440.
Article6. Raja SN, Haythornthwaite JA, Pappagallo M, Clark MR, Travison TG, Sabeen S, et al. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2002; 59:1015–1021. PMID: 12370455.
Article7. Wu CL, Tella P, Staats PS, Vaslav R, Kazim DA, Wesselmann U, et al. Analgesic effects of intravenous lidocaine and morphine on postamputation pain: a randomized doubleblind, active placebo-controlled, crossover trial. Anesthesiology. 2002; 96:841–848. PMID: 11964590.
Article8. Dobecki DA, Schocket SM, Wallace MS. Update on pharmacotherapy guidelines for the treatment of neuropathic pain. Curr Pain Headache Rep. 2006; 10:185–190. PMID: 18778572.
Article9. Rainsford KD. Current status of the therapeutic uses and actions of the preferential cyclo-oxygenase-2 NSAID, nimesulide. Inflammopharmacology. 2006; 14:120–137. PMID: 16983492.
Article10. Bianchi M, Broggini M. Anti-hyperalgesic effects of nimesulide: studies in rats and humans. Int J Clin Pract Suppl. 2002; (128):11–19. PMID: 12166613.11. Binning A. Nimesulide in the treatment of postoperative pain: a double-blind, comparative study in patients undergoing arthroscopic knee surgery. Clin J Pain. 2007; 23:565–570. PMID: 17710005.
Article12. Bianchi M, Broggini M, Balzarini P, Franchi S, Sacerdote P. Effects of nimesulide on pain and on synovial fluid concentrations of substance P, interleukin-6 and interleukin-8 in patients with knee osteoarthritis: comparison with celecoxib. Int J Clin Pract. 2007; 61:1270–1277. PMID: 17590218.
Article13. Bennett A, Villa G. Nimesulide: an NSAID that preferentially inhibits COX-2, and has various unique pharmacological activities. Expert Opin Pharmacother. 2000; 1:277–286. PMID: 11249549.
Article14. Durrieu G, Olivier P, Bagheri H, Montastruc JL;. Overview of adverse reactions to nefopam: an analysis of the French Pharmacovigilance database. Fundam Clin Pharmacol. 2007; 21:555–558. PMID: 17868209.
Article15. Girard P, Pansart Y, Coppe MC, Gillardin JM. Nefopam reduces thermal hypersensitivity in acute and postoperative pain models in the rat. Pharmacol Res. 2001; 44:541–545. PMID: 11735363.
Article16. Girard P, Coppé MC, Verniers D, Pansart Y, Gillardin JM. Role of catecholamines and serotonin receptor subtypes in nefopam-induced antinociception. Pharmacol Res. 2006; 54:195–202. PMID: 16750379.
Article17. Girard P, Pansart Y, Gillardin JM. Nefopam potentiates morphine antinociception in allodynia and hyperalgesia in the rat. Pharmacol Biochem Behav. 2004; 77:695–703. PMID: 15099914.
Article18. Beloeil H, Delage N, Nègre I, Mazoit JX, Benhamou D. The median effective dose of nefopam and morphine administered intravenously for postoperative pain after minor surgery: a prospective randomized double-blinded isobolographic study of their analgesic action. Anesth Analg. 2004; 98:395–400. PMID: 14742377.
Article19. Kapfer B, Alfonsi P, Guignard B, Sessler DI, Chauvin M. Nefopam and ketamine comparably enhance postoperative analgesia. Anesth Analg. 2005; 100:169–174. PMID: 15616073.
Article20. Biella GE, Groppetti A, Novelli A, Fernández-Sánchez MT, Manfredi B, Sotgiu ML. Neuronal sensitization and its behavioral correlates in a rat model of neuropathy are prevented by a cyclic analog of orphenadrine. J Neurotrauma. 2003; 20:593–601. PMID: 12906743.
Article21. Novelli A, Groppetti A, Rossoni G, Manfredi B, Ferrero-Gutiérrez A, Pérez-Gómez A, et al. Nefopam is more potent than carbamazepine for neuroprotection against veratridine in vitro and has anticonvulsant properties against both electrical and chemical stimulation. Amino Acids. 2007; 32:323–332. PMID: 17021653.
Article22. Backonja MM, Irving G, Argoff C. Rational multidrug therapy in the treatment of neuropathic pain. Curr Pain Headache Rep. 2006; 10:34–38. PMID: 16499828.
Article23. Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983; 16:109–110. PMID: 6877845.
Article24. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988; 33:87–107. PMID: 2837713.
Article25. Zhao C, Tall JM, Meyer RA, Raja SN. Antiallodynic effects of systemic and intrathecal morphine in the spared nerve injury model of neuropathic pain in rats. Anesthesiology. 2004; 100:905–911. PMID: 15087626.
Article26. Stuesse SL, Crisp T, McBurney DL, Schechter JB, Lovell JA, Cruce WL. Neuropathic pain in aged rats: behavioral responses and astrocytic activation. Exp Brain Res. 2001; 137:219–227. PMID: 11315551.
Article27. Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994; 59:369–376. PMID: 7708411.
Article28. Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003; 60:1524–1534. PMID: 14623723.29. Hamidi GA, Manaheji H, Janahmadi M, Noorbakhsh SM, Salami M. Co-administration of MK-801 and morphine attenuates neuropathic pain in rat. Physiol Behav. 2006; 88:628–635. PMID: 16815501.
Article30. Erichsen HK, Hao JX, Xu XJ, Blackburn-Munro G. Comparative actions of the opioid analgesics morphine, methadone and codeine in rat models of peripheral and central neuropathic pain. Pain. 2005; 116:347–358. PMID: 15982817.
Article31. Tassorelli C, Greco R, Sandrini G, Nappi G. Central components of the analgesic/antihyperalgesic effect of nimesulide: studies in animal models of pain and hyperalgesia. Drugs. 2003; 63(Suppl 1):9–22. PMID: 14506907.
Article32. Baillie JK, Power I. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005; 352:2650–2651. PMID: 15972877.
Article33. Nayebi AR, Hassanpour M, Rezazadeh H. Effect of chronic and acute administration of fluoxetine and its additive effect with morphine on the behavioural response in the formalin test in rats. J Pharm Pharmacol. 2001; 53:219–225. PMID: 11273019.
Article34. Ho KY, Tay W, Yeo MC, Liu H, Yeo SJ, Chia SL, et al. Duloxetine reduces morphine requirements after knee replacement surgery. Br J Anaesth. 2010; 105:371–376. PMID: 20573635.
Article35. Girard P, Niedergang B, Pansart Y, Coppé MC, Verleye M. Systematic evaluation of the nefopam-paracetamol combination in rodent models of antinociception. Clin Exp Pharmacol Physiol. 2011; [in press].
Article36. Girard P, Verniers D, Coppé MC, Pansart Y, Gillardin JM. Nefopam and ketoprofen synergy in rodent models of antinociception. Eur J Pharmacol. 2008; 584:263–271. PMID: 18316069.
Article37. Miranda HF, Lemus I, Pinardi G. Effect of the inhibition of serotonin biosynthesis on the antinociception induced by nonsteroidal anti-inflammatory drugs. Brain Res Bull. 2003; 61:417–425. PMID: 12909285.
Article38. Miranda HF, Sierralta F, Pinardi G. An isobolographic analysis of the adrenergic modulation of diclofenac antinociception. Anesth Analg. 2001; 93:430–435. PMID: 11473875.
Article39. Sandrini G, Tassorelli C, Cecchini AP, Alfonsi E, Nappi G. Effects of nimesulide on nitric oxide-induced hyperalgesia in humans--a neurophysiological study. Eur J Pharmacol. 2002; 450:259–262. PMID: 12208318.
Article40. Fletcher D, Benoist JM, Gautron M, Guilbaud G. Isobolographic analysis of interactions between intravenous morphine, propacetamol, and diclofenac in carrageenin-injected rats. Anesthesiology. 1997; 87:317–326. PMID: 9286896.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antinociceptive Effect of Intrathecal Nefopam and Interaction with Morphine in Formalin-Induced Pain of Rats
- Use of Nefopam in Perioperative Pain Management; Keeping Nefopam in between
- The Potential Role of Intrathecal Nefopam in the Management of Neuropathic Pain
- Interaction between Neostigmine and Morphine in the Neuropathic Pain Model
- Nefopam Reduces Dysesthesia after Percutaneous Endoscopic Lumbar Discectomy