Korean J Pain.
2014 Oct;27(4):326-333. 10.3344/kjp.2014.27.4.326.
Effects of Nefopam on Streptozotocin-Induced Diabetic Neuropathic Pain in Rats
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. choiss@amc.seoul.kr
- 2Department of Anesthesiology and Pain Medicine, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea.
- KMID: 1802498
- DOI: http://doi.org/10.3344/kjp.2014.27.4.326
Abstract
- BACKGROUND
Nefopam is a centrally acting non-opioid analgesic agent. Its analgesic properties may be related to the inhibitions of monoamine reuptake and the N-methyl-D-aspartate (NMDA) receptor. The antinociceptive effect of nefopam has been shown in animal models of acute and chronic pain and in humans. However, the effect of nefopam on diabetic neuropathic pain is unclear. Therefore, we investigated the preventive effect of nefopam on diabetic neuropathic pain induced by streptozotocin (STZ) in rats.
METHODS
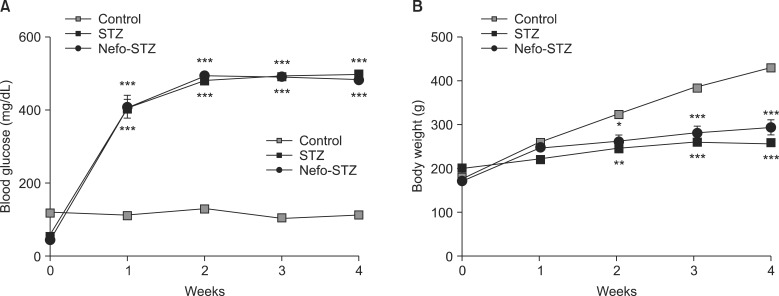
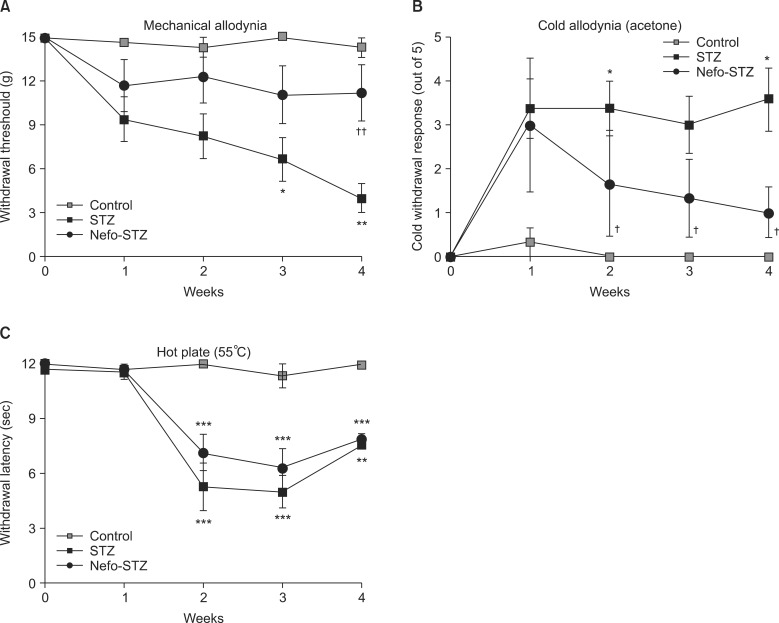
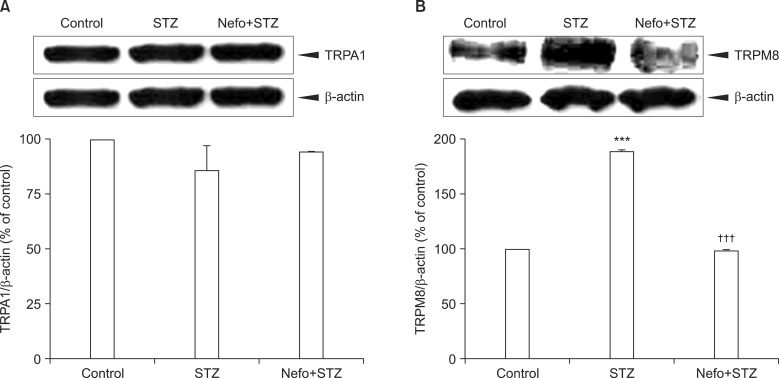
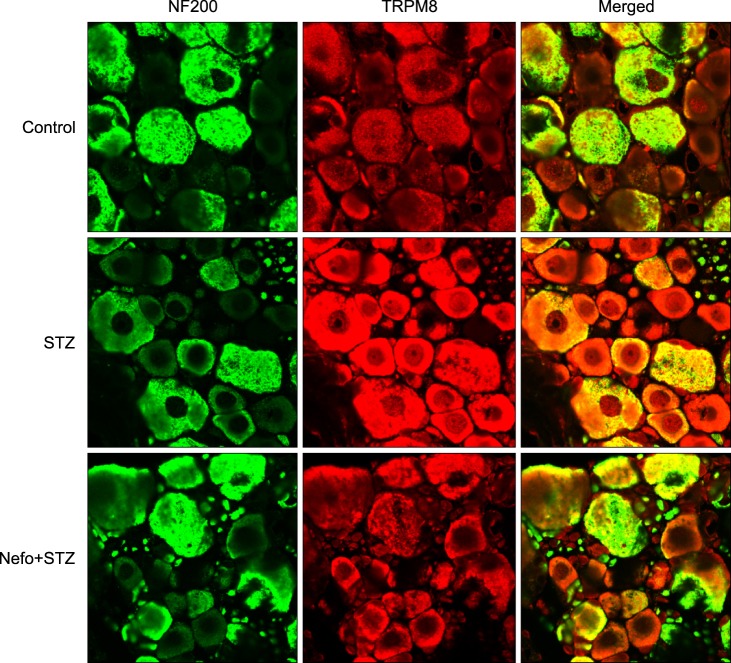
Pretreatment with nefopam (30 mg/kg) was performed intraperitoneally 30 min prior to an intraperitoneal injection of STZ (60 mg/kg). Mechanical and cold allodynia were tested before, and 1 to 4 weeks after drug administration. Thermal hyperalgesia was also investigated. In addition, the transient receptor potential ankyrin 1 (TRPA1) and TRP melastatin 8 (TRPM8) expression levels in the dorsal root ganglion (DRG) were evaluated.
RESULTS
Pretreatment with nefopam significantly inhibited STZ-induced mechanical and cold allodynia, but not thermal hyperalgesia. The STZ injection increased TRPM8, but not TRPA1, expression levels in DRG neurons. Pretreatment with nefopam decreased STZ-induced TRPM8 expression levels in the DRG.
CONCLUSIONS
These results demonstrate that a nefopam pretreatment has strong antiallodynic effects on STZ-induced diabetic rats, which may be associated with TRPM8 located in the DRG.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Increased calcium-mediated cerebral processes after peripheral injury: possible role of the brain in complex regional pain syndrome
Francis Sahngun Nahm, Jae-Sung Lee, Pyung-Bok Lee, Eunjoo Choi, Woong Ki Han, Sang-Soep Nahm
Korean J Pain. 2020;33(2):131-137. doi: 10.3344/kjp.2020.33.2.131.An Update on the Management of Diabetic Neuropathic Pain: A Few Comments
Mohamed Amin Ghobadifar
Korean J Pain. 2015;28(2):158-159. doi: 10.3344/kjp.2015.28.2.158.
Reference
-
1. Durrieu G, Olivier P, Bagheri H, Montastruc JL. French Network of Pharmacovigilance Centers. Overview of adverse reactions to nefopam: an analysis of the French Pharmacovigilance database. Fundam Clin Pharmacol. 2007; 21:555–558. PMID: 17868209.
Article2. Girard P, Coppé MC, Verniers D, Pansart Y, Gillardin JM. Role of catecholamines and serotonin receptor subtypes in nefopam-induced antinociception. Pharmacol Res. 2006; 54:195–202. PMID: 16750379.
Article3. Verleye M, André N, Heulard I, Gillardin JM. Nefopam blocks voltage-sensitive sodium channels and modulates glutamatergic transmission in rodents. Brain Res. 2004; 1013:249–255. PMID: 15193535.
Article4. Girard P, Pansart Y, Coppe MC, Gillardin JM. Nefopam reduces thermal hypersensitivity in acute and postoperative pain models in the rat. Pharmacol Res. 2001; 44:541–545. PMID: 11735363.
Article5. Biella GE, Groppetti A, Novelli A, Fernández-Sánchez MT, Manfredi B, Sotgiu ML. Neuronal sensitization and its behavioral correlates in a rat model of neuropathy are prevented by a cyclic analog of orphenadrine. J Neurotrauma. 2003; 20:593–601. PMID: 12906743.
Article6. Saghaei E, Moini Zanjani T, Sabetkasaei M, Naseri K. Enhancement of antinociception by co-administrations of nefopam, morphine, and nimesulide in a rat model of neuropathic pain. Korean J Pain. 2012; 25:7–15. PMID: 22259710.
Article7. Schnedl WJ, Ferber S, Johnson JH, Newgard CB. STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes. 1994; 43:1326–1333. PMID: 7926307.
Article8. Sima AA, Sugimoto K. Experimental diabetic neuropathy: an update. Diabetologia. 1999; 42:773–788. PMID: 10440118.
Article9. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997; 389:816–824. PMID: 9349813.
Article10. Brederson JD, Kym PR, Szallasi A. Targeting TRP channels for pain relief. Eur J Pharmacol. 2013; 716:61–76. PMID: 23500195.
Article11. Wei H, Hämäläinen MM, Saarnilehto M, Koivisto A, Pertovaara A. Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiology. 2009; 111:147–154. PMID: 19512877.
Article12. Koivisto A, Hukkanen M, Saarnilehto M, Chapman H, Kuokkanen K, Wei H, et al. Inhibiting TRPA1 ion channel reduces loss of cutaneous nerve fiber function in diabetic animals: sustained activation of the TRPA1 channel contributes to the pathogenesis of peripheral diabetic neuropathy. Pharmacol Res. 2012; 65:149–158. PMID: 22133672.
Article13. Wei H, Chapman H, Saarnilehto M, Kuokkanen K, Koivisto A, Pertovaara A. Roles of cutaneous versus spinal TRPA1 channels in mechanical hypersensitivity in the diabetic or mustard oil-treated non-diabetic rat. Neuropharmacology. 2010; 58:578–584. PMID: 20004676.
Article14. Su L, Wang C, Yu YH, Ren YY, Xie KL, Wang GL. Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci. 2011; 12:120. PMID: 22111979.
Article15. Xing H, Chen M, Ling J, Tan W, Gu JG. TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci. 2007; 27:13680–13690. PMID: 18077679.
Article16. Talbot S, Chahmi E, Dias JP, Couture R. Key role for spinal dorsal horn microglial kinin B1 receptor in early diabetic pain neuropathy. J Neuroinflammation. 2010; 7:36. PMID: 20587056.17. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63. PMID: 7990513.
Article18. Kim SH, Nam JS, Choi DK, Koh WW, Suh JH, Song JG, et al. Tumor necrosis factor-alpha and apoptosis following spinal nerve ligation injury in rats. Korean J Pain. 2011; 24:185–190. PMID: 22220239.
Article19. Li Y, Dorsi MJ, Meyer RA, Belzberg AJ. Mechanical hyperalgesia after an L5 spinal nerve lesion in the rat is not dependent on input from injured nerve fibers. Pain. 2000; 85:493–502. PMID: 10781924.
Article20. Choi SS, Koh WU, Nam JS, Shin JW, Leem JG, Suh JH. Effect of ethyl pyruvate on Paclitaxel-induced neuropathic pain in rats. Korean J Pain. 2013; 26:135–141. PMID: 23614074.
Article21. Laboureyras E, Chateauraynaud J, Richebé P, Simonnet G. Long-term pain vulnerability after surgery in rats: prevention by nefopam, an analgesic with antihyperalgesic properties. Anesth Analg. 2009; 109:623–631. PMID: 19608840.
Article22. Sigaudo-Roussel D, Fromy B, Saumet JL. Diabetic neuropathy in animal models. Drug Discov Today Dis Models. 2007; 4:39–44.
Article23. Leurs R, Donnell D, Timmerman H, Bast A. Cytochrome P450 metabolic intermediate complex of nefopam. J Pharm Pharmacol. 1987; 39:835–837. PMID: 2891822.
Article24. Barrière DA, Rieusset J, Chanteranne D, Busserolles J, Chauvin MA, Chapuis L, et al. Paclitaxel therapy potentiates cold hyperalgesia in streptozotocin-induced diabetic rats through enhanced mitochondrial reactive oxygen species production and TRPA1 sensitization. Pain. 2012; 153:553–561. PMID: 22177224.
Article25. Wrigley PJ, Jeong HJ, Vaughan CW. Primary afferents with TRPM8 and TRPA1 profiles target distinct subpopulations of rat superficial dorsal horn neurones. Br J Pharmacol. 2009; 157:371–380. PMID: 19371346.
Article26. Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain. 2010; 150:340–350. PMID: 20542379.
Article27. Colburn RW, Lubin ML, Stone DJ Jr, Wang Y, Lawrence D, D'Andrea MR, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007; 54:379–386. PMID: 17481392.
Article28. Klein AH, Sawyer CM, Carstens MI, Tsagareli MG, Tsiklauri N, Carstens E. Topical application of L-menthol induces heat analgesia, mechanical allodynia, and a biphasic effect on cold sensitivity in rats. Behav Brain Res. 2010; 212:179–186. PMID: 20398704.
Article29. Namer B, Seifert F, Handwerker HO, Maihöfner C. TRPA1 and TRPM8 activation in humans: effects of cinnamaldehyde and menthol. Neuroreport. 2005; 16:955–959. PMID: 15931068.
Article30. Calcutt NA. Potential mechanisms of neuropathic pain in diabetes. Int Rev Neurobiol. 2002; 50:205–228. PMID: 12198811.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Proliferation of Cultured Vascular Smooth Muscle Cells(VSMCs) Obtained from Aortas of Insulin Dependent Diabetic Rats
- Use of Nefopam in Perioperative Pain Management; Keeping Nefopam in between
- Enhancement of Antinociception by Co-administrations of Nefopam, Morphine, and Nimesulide in a Rat Model of Neuropathic Pain
- The Potential Role of Intrathecal Nefopam in the Management of Neuropathic Pain
- Intravenous Nefopam Reduces Postherpetic Neuralgia during the Titration of Oral Medications