Korean J Physiol Pharmacol.
2010 Dec;14(6):365-369. 10.4196/kjpp.2010.14.6.365.
Melatonin Induces Apoptotic Cell Death via p53 in LNCaP Cells
- Affiliations
-
- 1Department of Biomedical Engineering, College of Health Science, Yonsei University, Wonju 220-710, Korea. yooym@yonsei.ac.kr
- KMID: 2071707
- DOI: http://doi.org/10.4196/kjpp.2010.14.6.365
Abstract
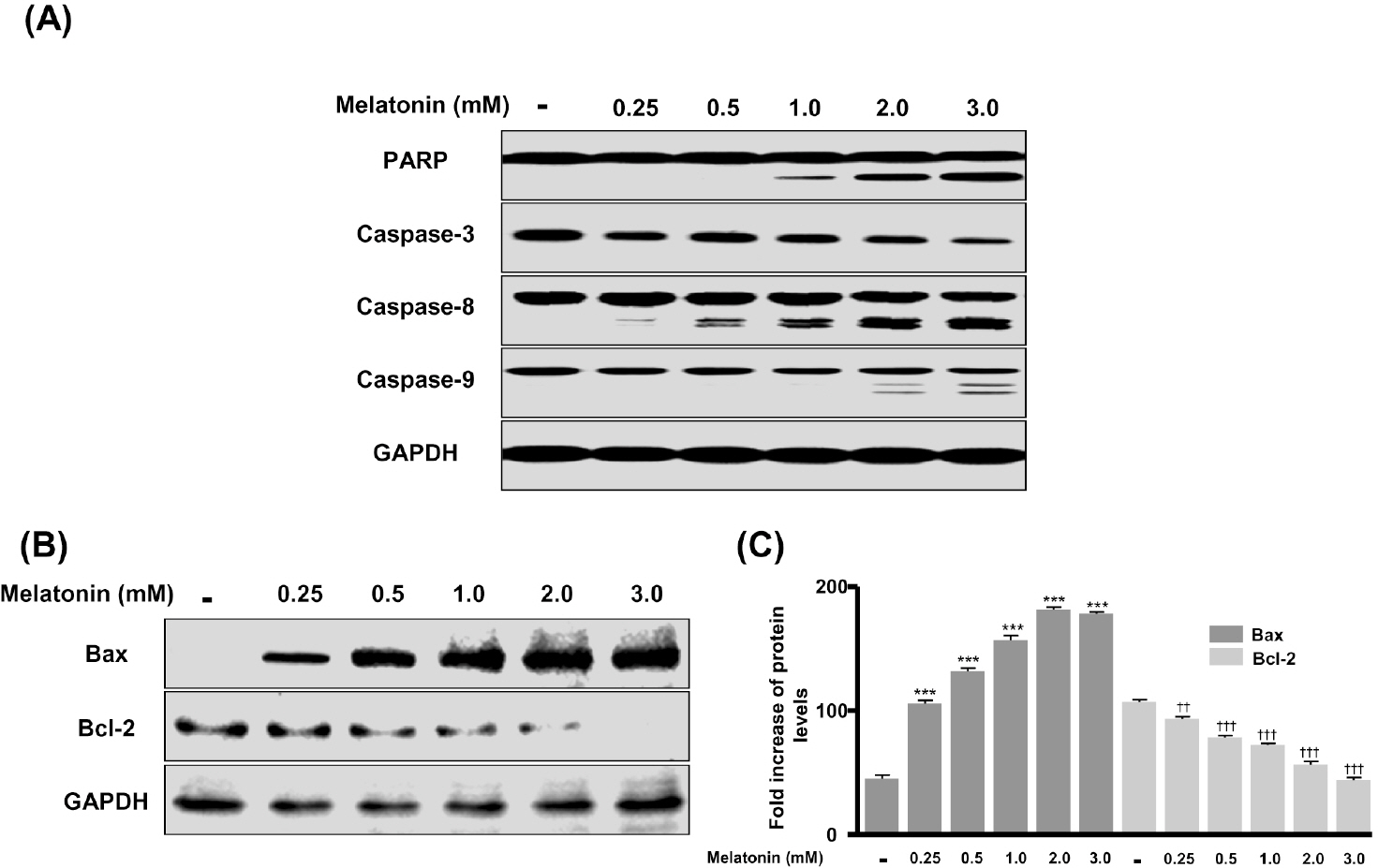
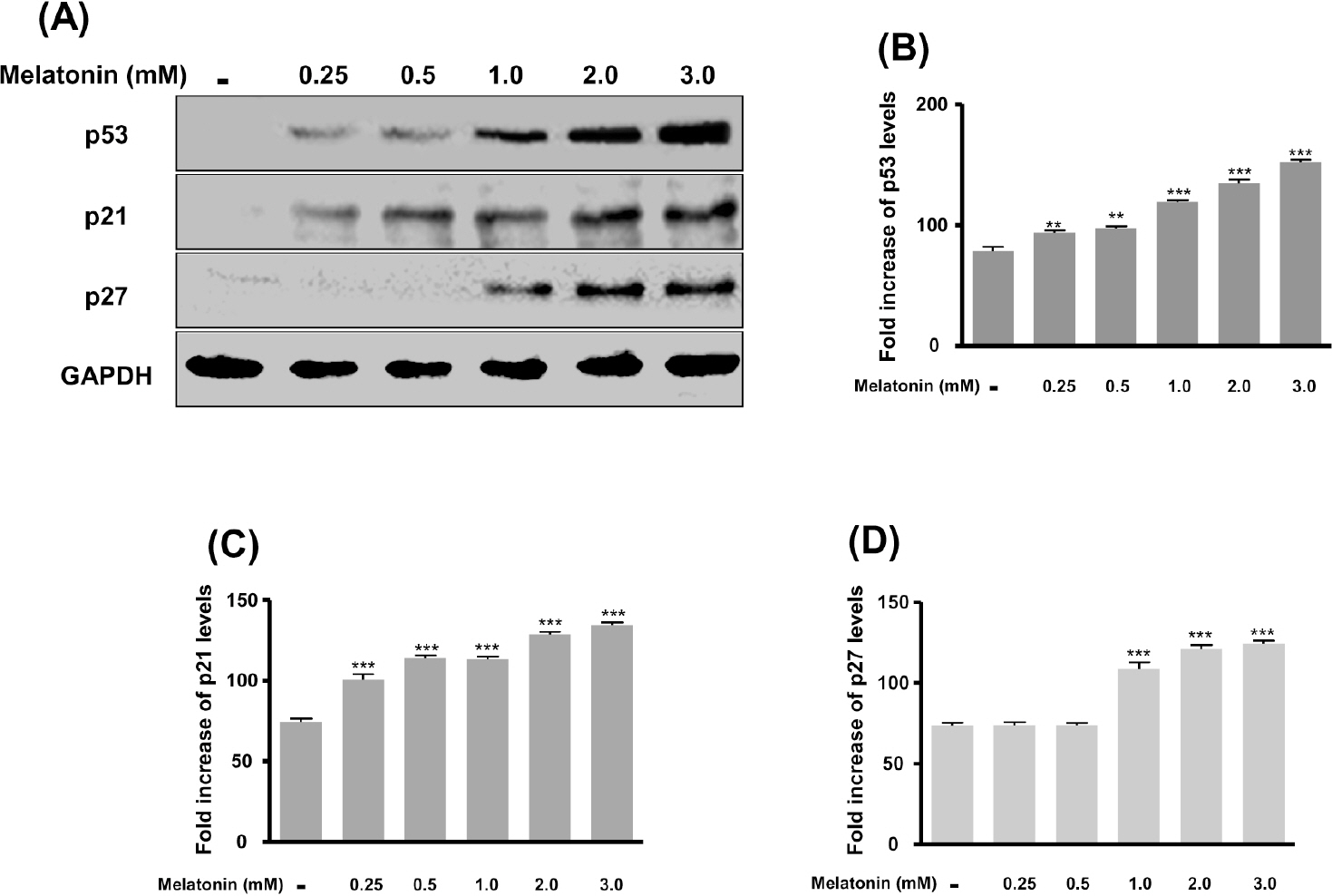
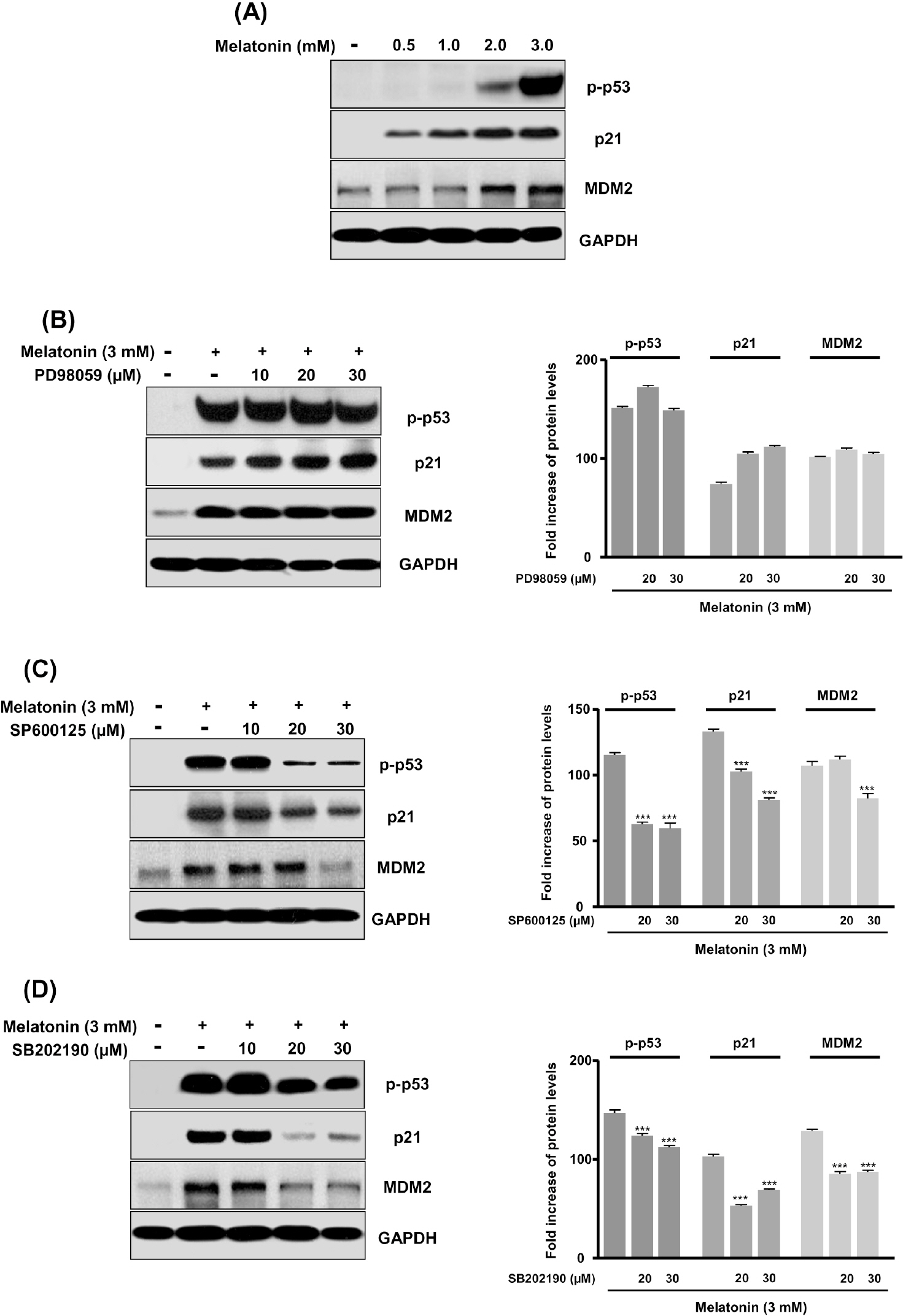
- In this study, we examined whether melatonin promotes apoptotic cell death via p53 in prostate LNCaP cells. Melatonin treatment significantly curtailed the growth of LNCaP cells in a dose- and time-dependent manner. Melatonin treatment (0 to 3 mM) induced the fragmentation of poly(ADP-ribose) polymerase (PARP) and activation of caspase-3, caspase-8, and caspase-9. Moreover, melatonin markedly activated Bax expression and decreased Bcl-2 expression in dose increments. To investigate p53 and p21 expression, LNCaP cells were treated with 0 to 3 mM melatonin. Melatonin increased the expressions of p53, p21, and p27. Treatment with mitogen-activated protein kinase (MAPK) inhibitors, PD98059 (ERK inhibitor), SP600125 (JNK inhibitor) and SB202190 (p38 inhibitor), confirmed that the melatonin-induced apoptosis was p21-dependent, but ERK-independent. With the co-treatment of PD98059 and melatonin, the expression of p-p53, p21, and MDM2 did not decrease. These effects were opposite to the expression of p-p53, p21, and MDM2 observed with SP600125 and SB202190 treatments. Together, these results suggest that p53-dependent induction of JNK/p38 MAPK directly participates in apoptosis induced by melatonin.
Keyword
- Melatonin; p53; p38; JNK; LNCaP cells
MeSH Terms
Figure
Cited by 1 articles
-
Increased HoxB4 Inhibits Apoptotic Cell Death in Pro-B Cells
Sung-Won Park, Kyung-Jong Won, Yong-Soo Lee, Hye Sun Kim, Yu-Kyung Kim, Hyeon-Woo Lee, Bokyung Kim, Byeong Han Lee, Jin-Hoi Kim, Dong-Ku Kim
Korean J Physiol Pharmacol. 2012;16(4):265-271. doi: 10.4196/kjpp.2012.16.4.265.
Reference
-
References
1. Ben Sahra I, Laurent K, Giuliano S, Larbret F, Ponzio G, Gounon P, Le Marchand-Brustel Y, Giorgetti-Peraldi S, Cormont M, Bertolotto C, Deckert M, Auberger P, Tanti JF, Bost F. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010; 70:2465–2475.
Article2. Yu CX, Zhang XQ, Kang LD, Zhang PJ, Chen WW, Liu WW, Zhang JY. Emodin induces apoptosis in human prostate cancer cell LNCaP. Asian J Androl. 2008; 10:625–634.
Article3. Lee DH, Kim C, Zhang L, Lee YJ. Role of p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer cells. Biochem Pharmacol. 2008; 75:2020–2033.
Article4. Logan IR, McNeill HV, Cook S, Lu X, Lunec J, Robson CN. Analysis of the MDM2 antagonist nutlin-3 in human prostate cancer cells. Prostate. 2007; 67:900–906.
Article5. Sainz RM, Mayo JC, Tan DX, León J, Manchester L, Reiter RJ. Melatonin reduces prostate cancer cell growth leading to neuroendocrine differentiation via a receptor and PKA independent mechanism. Prostate. 2005; 63:29–43.
Article6. Tam CW, Mo CW, Yao KM, Shiu SY. Signaling mechanisms of melatonin in antiproliferation of hormone-refractory 22Rv1 human prostate cancer cells: implications for prostate cancer chemoprevention. J Pineal Res. 2007; 42:191–202.
Article7. Cucina A, Proietti S, D'Anselmi F, Coluccia P, Dinicola S, Frati L, Bizzarri M. Evidence for a biphasic apoptotic pathway induced by melatonin in MCF-7 breast cancer cells. J Pineal Res. 2009; 46:172–180.
Article8. Martín-Renedo J, Mauriz JL, Jorquera F, Ruiz-Andrés O, González P, González-Gallego J. Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J Pineal Res. 2008; 45:532–540.
Article9. Xi SC, Tam PC, Brown GM, Pang SF, Shiu SY. Potential involvement of MT1 receptor and attenuated sex steroid induced calcium influx in the direct anti-proliferative action of melatonin on androgen-responsive LNCaP human prostate cancer cells. J Pineal Res. 2000; 29:172–183.10. Lupowitz Z, Zisapel N. Hormonal interactions in human prostate tumor LNCaP cells. J Steroid Biochem Mol Biol. 1999; 68:83–88.
Article11. Moretti RM, Marelli MM, Maggi R, Dondi D, Motta M, Limonta P. Antiproliferative action of melatonin on human prostate cancer LNCaP cells. Oncol Rep. 2000; 7:347–351.
Article12. Xi SC, Siu SWF, Fong SW, Shiu SYW. Inhibition of androgen sensitive LNCaP prostate cancer growth in vivo by melatonin: association of antiproliferative action of the pineal hormone with MT1 receptor protein expression. Prostate. 2001; 46:52–61.13. Joo SS, Yoo YM. Melatonin induces apoptotic death in LNCaP cells via p38 and JNK pathways: therapeutic implications for prostate cancer. J Pineal Res. 2009; 47:8–14.
Article14. Galluzzi L, Morselli E, Kepp O, Tajeddine N, Kroemer G. Targeting p53 to mitochondria for cancer therapy. Cell Cycle. 2008; 7:1949–1955.
Article15. Califice S, Waltregny D, Castronovo V, van den Brûle F. Prostate carcinoma cell lines and apoptosis: a review. Rev Med Liege. 2004; 59:704–710.16. Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997; 91:325–334.
Article17. Kim YS, Lee HJ, Jang C, Kim HS, Cho YJ. Knockdown of RCAN1.4 Increases Susceptibility to FAS-mediated and DNA-damage-induced Apoptosis by Upregulation of p53 Expression. Korean J Physiol Pharmacol. 2009; 13:483–489.
Article18. Bulavin DV, Saito S, Hollander MC, Sakaguchi K, Anderson CW, Appella E, Fornace AJ Jr. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 1999; 18:6845–6854.
Article19. Huang C, Ma WY, Maxiner A, Sun Y, Dong Z. p38 kinase mediates UV-induced phosphorylation of p53 protein at serine 389. J Biol Chem. 1999; 274:12229–12235.
Article20. She QB, Chen N, Dong Z. ERKs and p38 kinase phosphorylate p53 protein at serine 15 in response to UV radiation. J Biol Chem. 2000; 275:20444–20449.
Article21. Sayed M, Kim SO, Salh BS, Issinger OG, Pelech SL. Stress-induced activation of protein kinase CK2 by direct interaction with p38 mitogen-activated protein kinase. J Biol Chem. 2000; 275:16569–16573.
Article22. Hu MC, Qiu WR, Wang YP. JNK1, JNK2 and JNK3 are p53 N-terminal serine 34 kinases. Oncogene. 1997; 15:2277–2287.
Article23. Kwon YW, Ueda S, Ueno M, Yodoi J, Masutani H. Mechanism of p53-dependent apoptosis induced by 3-methylcholanthrene. J Biol Chem. 2002; 277:1837–1844.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Study on the Mitochondrial Dysfunction by p53 Regulation in Ceramide-induced Neuronal Cell Death
- Molecular Aspects of Radiotherapy
- Melatonin Protects Human Adipose-Derived Stem Cells from Oxidative Stress and Cell Death
- Influence of Estrogen and Polyamines on Mifepristone-induced Apoptosis in Prostate Cancer Cells
- Effects of Cyclooxygenase-2 on Prostatic Cancer Cell Lines