Korean J Physiol Pharmacol.
2009 Jun;13(3):229-239. 10.4196/kjpp.2009.13.3.229.
Provinol Inhibits Catecholamine Secretion from the Rat Adrenal Medulla
- Affiliations
-
- 1DNA Repair Research Center, Chosun University, Gwangju 501-759, Korea.
- 2Department of Pharmacology, College of Medicine, Chosun University, Gwangju 501-759, Korea. dylim@chosun.ac.kr
- KMID: 2071676
- DOI: http://doi.org/10.4196/kjpp.2009.13.3.229
Abstract
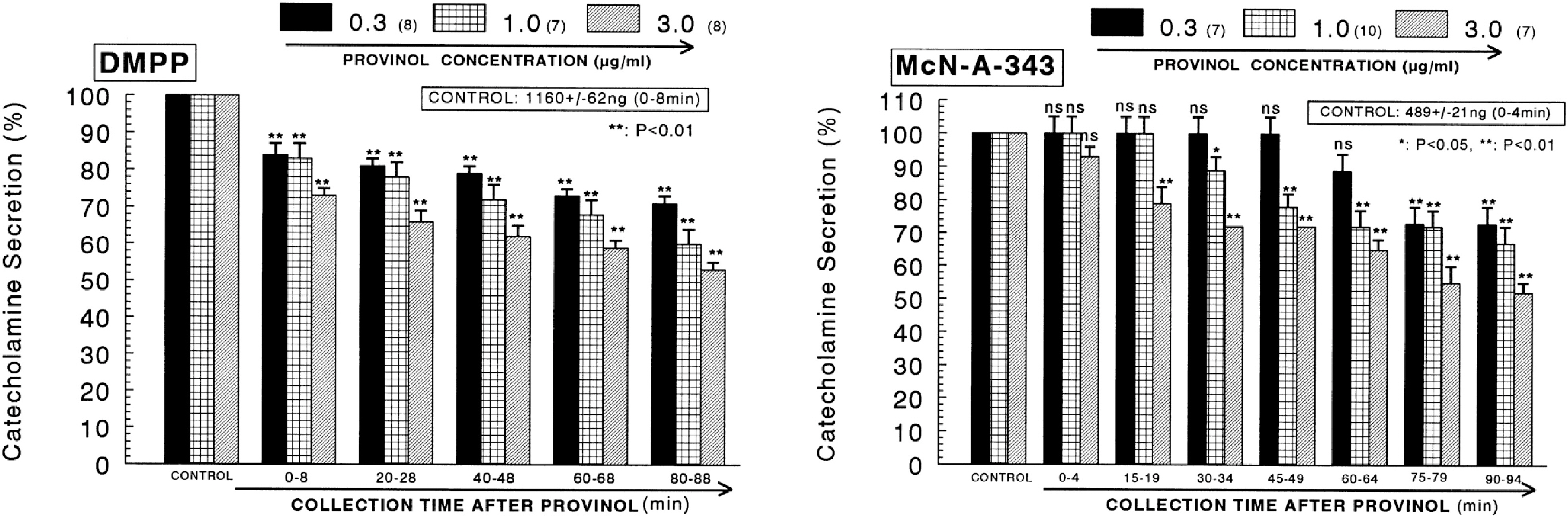
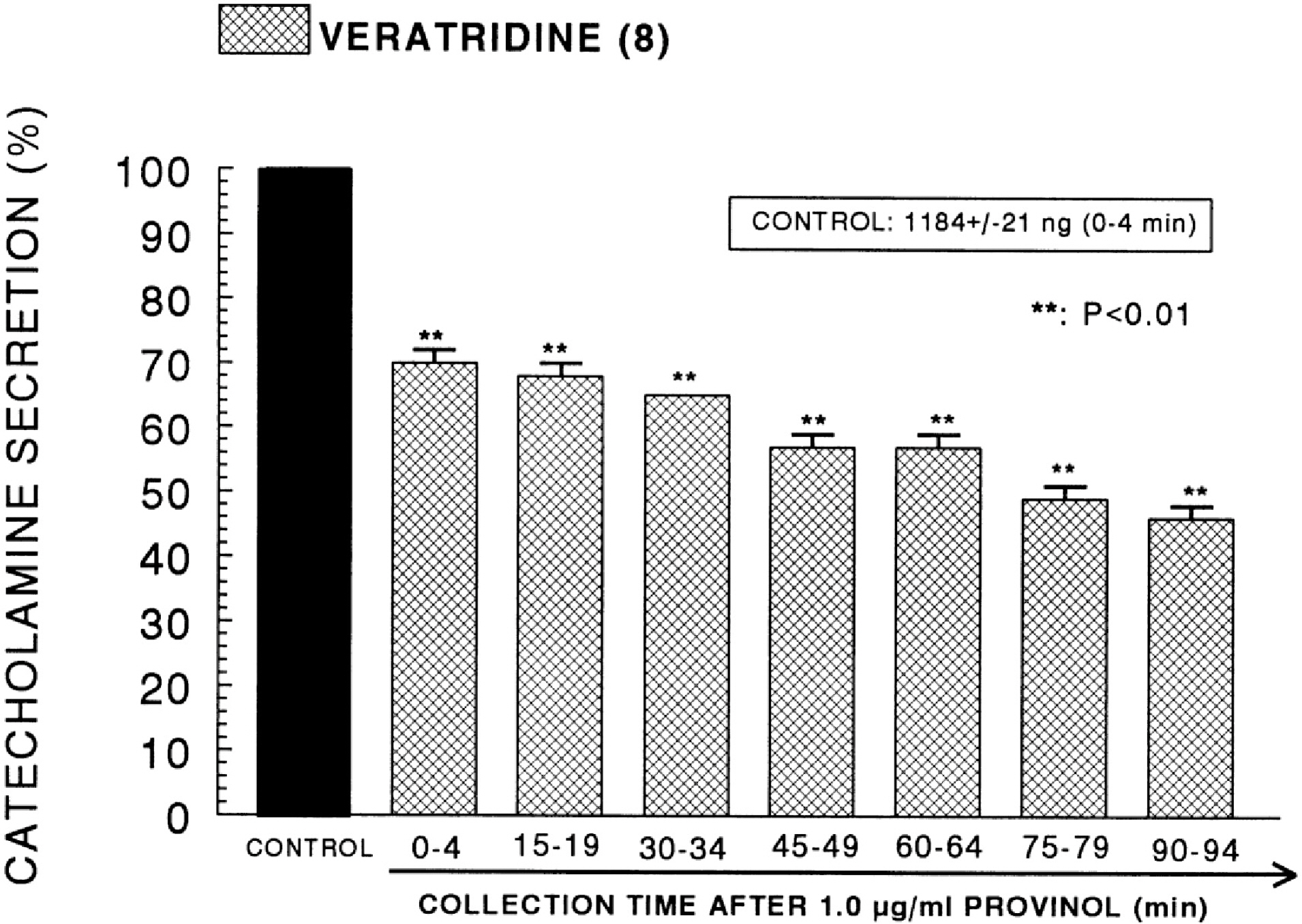
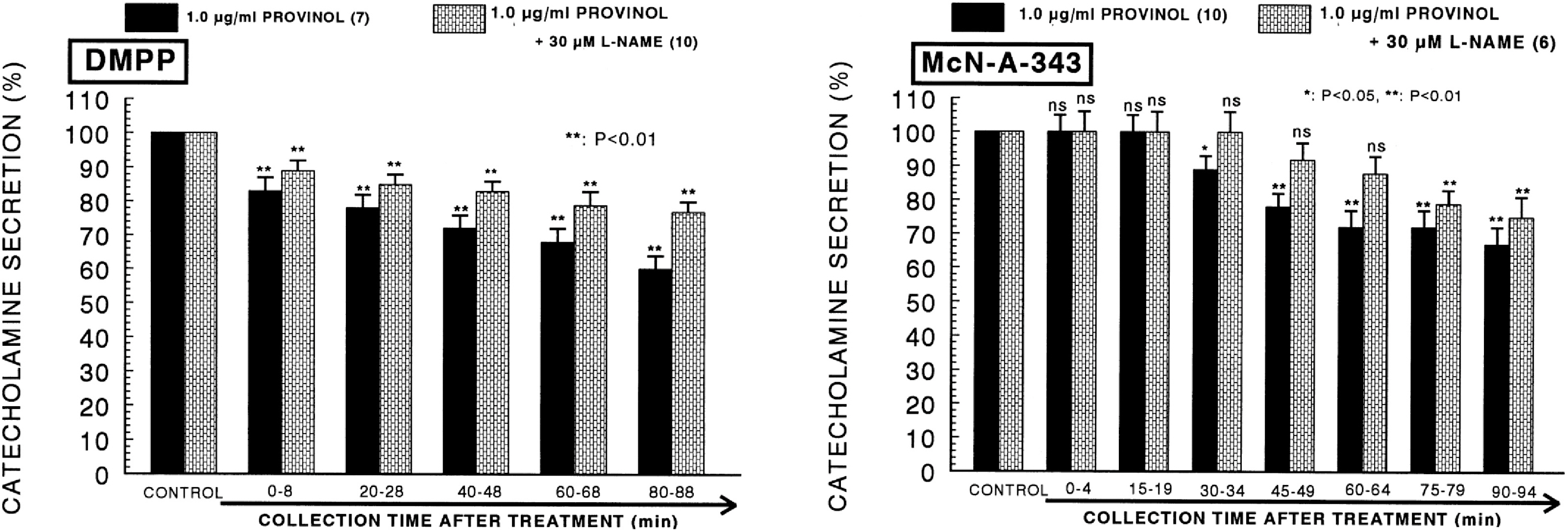
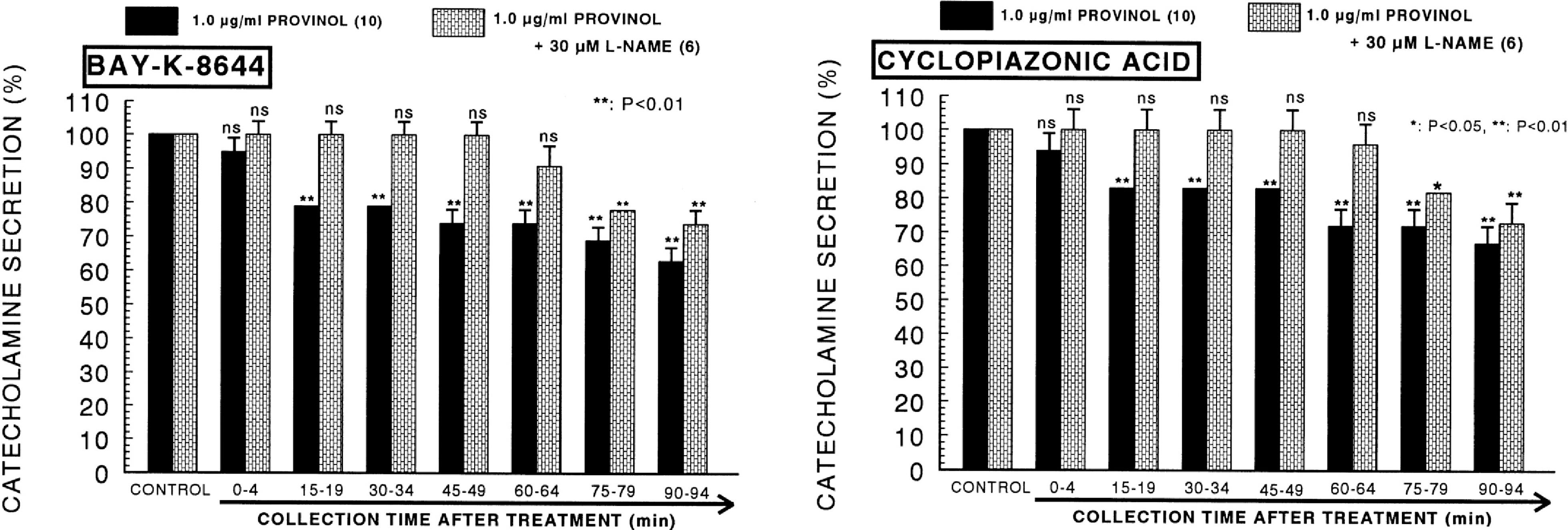
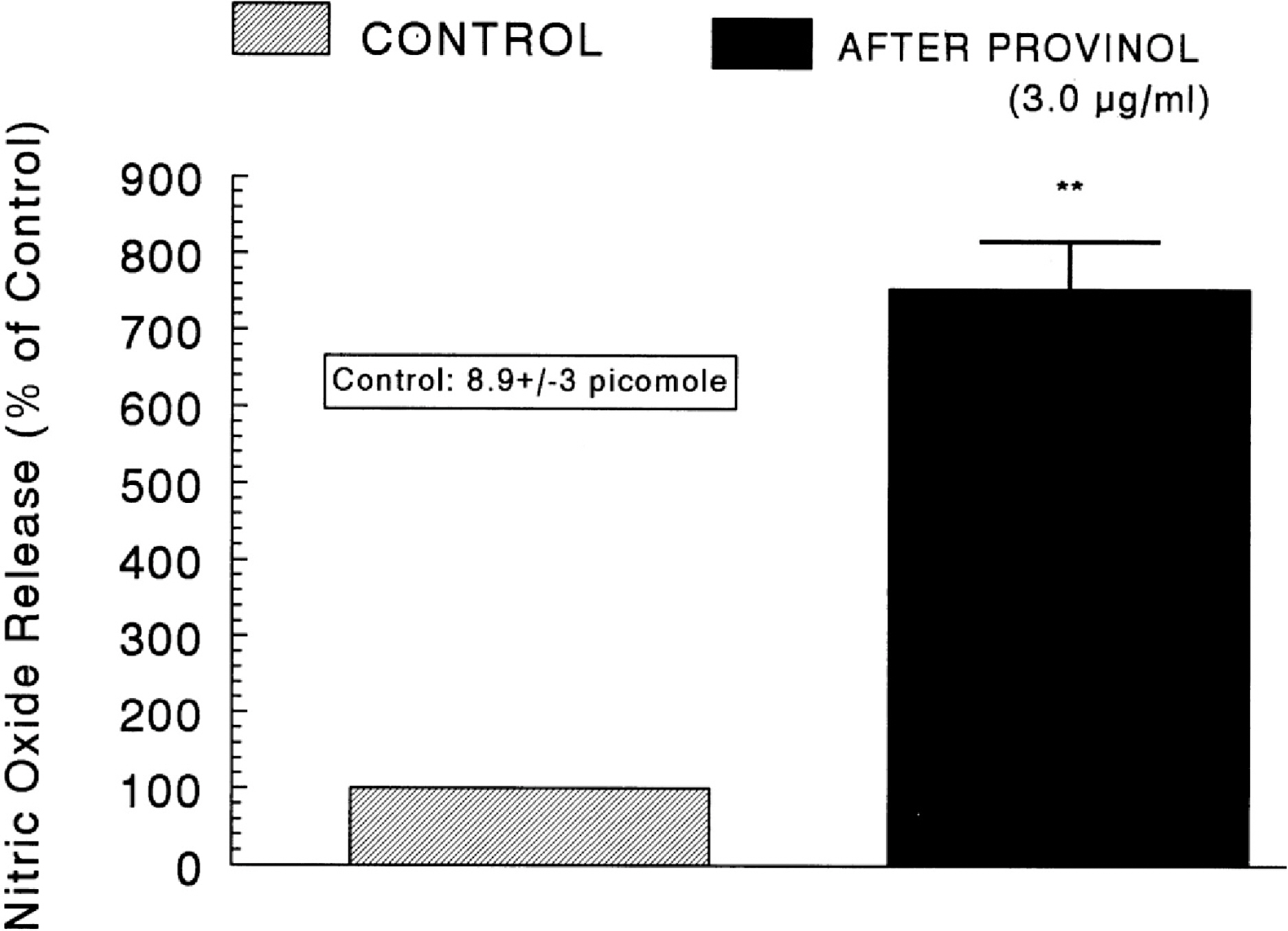
- The aim of the present study was to examine the effect of provinol, which is a mixture of polyphenolic compounds from red wine, on the secretion of catecholamines (CA) from isolated perfused rat adrenal medulla, and to elucidate its mechanism of action. Provinol (0.3~3 microgram/ml) perfused into an adrenal vein for 90 min dose- and time-dependently inhibited the CA secretory responses evoked by ACh (5.32 mM), high K+ (a direct membrane-depolarizer, 56 mM), DMPP (a selective neuronal nicotinic NN receptor agonist, 100 micrometer) and McN-A-343 (a selective muscarinic M1 receptor agonist, 100 micrometer). Provinol itself did not affect basal CA secretion. Also, in the presence of provinol (1 microgram/ml), the secretory responses of CA evoked by Bay-K-8644 (a voltage-dependent L-type dihydropyridine Ca2+ channel activator, 10 microgram), cyclopiazonic acid (a cytoplasmic Ca2+-ATPase inhibitor, 10 microgram) and veratridine (an activator of voltage-dependent Na+ channels, 10 microgram) were significantly reduced. Interestingly, in the simultaneous presence of provinol (1 microgram/ml) plus L-NAME (a selective inhibitor of NO synthase, 30 micrometer), the CA secretory responses evoked by ACh, high K+, DMPP, McN-A-343, Bay-K-8644 and cyclpiazonic acid recovered to the considerable extent of the corresponding control secretion in comparison with the inhibition of provinol-treatment alone. Under the same condition, the level of NO released from adrenal medulla after the treatment of provinol (3 microgram/ml) was greatly elevated in comparison to its basal release. Taken together, these data demonstrate that provinol inhibits the CA secretory responses evoked by stimulation of cholinergic (both muscarinic and nicotinic) receptors as well as by direct membrane-depolarization from the perfused rat adrenal medulla. This inhibitory effect of provinol seems to be exerted by inhibiting the influx of both calcium and sodium into the rat adrenal medullary cells along with the blockade of Ca2+ release from the cytoplasmic calcium store at least partly through the increased NO production due to the activation of nitric oxide synthase.
MeSH Terms
-
(4-(m-Chlorophenylcarbamoyloxy)-2-butynyl)trimethylammonium Chloride
3-Pyridinecarboxylic acid, 1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-(trifluoromethyl)phenyl)-, Methyl ester
Adrenal Medulla
Animals
Calcium
Catecholamines
Cytoplasm
Dihydropyridines
Dimethylphenylpiperazinium Iodide
Indoles
Neurons
NG-Nitroarginine Methyl Ester
Nitric Oxide
Nitric Oxide Synthase
Rats
Receptor, Muscarinic M1
Receptors, Cholinergic
Sodium
Veins
Veratridine
Wine
(4-(m-Chlorophenylcarbamoyloxy)-2-butynyl)trimethylammonium Chloride
3-Pyridinecarboxylic acid, 1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-(trifluoromethyl)phenyl)-, Methyl ester
Calcium
Catecholamines
Dihydropyridines
Dimethylphenylpiperazinium Iodide
Indoles
NG-Nitroarginine Methyl Ester
Nitric Oxide
Nitric Oxide Synthase
Receptor, Muscarinic M1
Receptors, Cholinergic
Sodium
Veratridine
Figure
Reference
-
Andriambeloson E., Kleschyov AL., Muller B., Beretz A., Stoclet JC., Andriantsitohaina R. Nitric oxide production and endothelium-dependent vasorelaxation induced by wine polyphenols in rat aorta. Br J Pharmacol. 120:1053–1058. 1997.
ArticleAndriambeloson E., Magnier C., Haan-Archipoff G., Lobstein A., Anton R., Beretz A., Stoclet JC., Andriantsitohaina R. Natural dietary polyphenolic compounds cause endothelium-dependent vasorelaxation in rat thoracic aorta. J Nutr. 128:2324–2333. 1998.
ArticleAndriambeloson E., Stoclet JC., Andriantsitohaina R. Mechanism of endothelial nitric oxide-dependent vasorelaxation induced by wine polyphenols in rat thoracic aorta. J Cardiovasc Pharmacol. 33:248–254. 1999.
ArticleAnton AH., Sayre DF. A study of the factors affecting the aluminum oxide trihydroxy indole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 138:360–375. 1962.Bernátová I., Púzserová A., Navarová J., Csizmadiová Z., Zeman M. Crowding-induced alterations in vascular system of Wistar-Kyoto rats: role of nitric oxide. Physiol Res. 56:667–669. 2007.
ArticleBreslow MJ., Tobin JR., Bredt DS., Ferris CD., Snyder SH., Traystman RJ. Nitric oxide as a regulator of adrenal blood flow. Am J Physiol (Heart Circ Physiol). 264:H464–H469. 1993.
ArticleBreslow MJ., Tobin JR., Bredt DS., Ferris CD., Snyder SH., Traystman RJ. Role of nitric oxide in adrenal medullary vasodilation during catecholamine secretion. Eur J Pharmacol. 210:105–106. 1992.
ArticleBurgoyne RD. Mechanism of secretion from adrenal chromaffin cells. Biochem Biophys Acta. 779:201–216. 1984.Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 26:13–25. 2000.Challiss RAJ., Jones JA., Owen PJ., Boarder MR. Changes in inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate mass accumulations in cultured adrenal chromaffin cells in response to bradykinin and histamine. J Neurochem. 56:1083–1086. 1991.
ArticleCheek TR., O'Sullivan AJ., Moreton RB., Berridge MJ., Burgoyne RD. Spatial localization of the stimulus-induced rise in cytosolic Ca2+ in bovine adrenal chromaffin cells: Distinct nicotinic and muscarinic patterns. FEBS Lett. 247:429–434. 1989.Chen CK., Pace-Asciak CR. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen Pharmacol. 27:363–366. 1996.Cishek MB., Galloway MT., Karim M., German JB., Kappagoda CT. Effects of red wine on endothelium dependent-relaxation in rabbits. Clin Sci. 93:507–511. 1997.Curin Y., Andriantsitohaina R. Polyphenols as potential therapeutical agents against cardiovascular diseases. Pharmacol Rep. 57:97–107. 2005.DeLorgeril M., Salen P., Martin JL., Mamelle N., Monjaud I., Touboul P., Delaye J. Effect of a Mediterranean type of diet on the rate of cardiovascular complications in patients with coronary artery disease. Insights into the protective effect of certain nutrients. J Am Coll Cardiol. 28:1103–1108. 1996.Demrow HS., Slane PR. Administration of wine and grape juice inhibits in vivo platelet activity and thrombosis in stenosed canine coronary arteries. Circulation. 91:1182–1188. 1995.
ArticleDiebolt M., Bucher B., Andriantsitohaina R. Wine polyphenols decrease blood pressure, improve NO vasodilatation, and induce gene expression. Hypertension. 38:159–165. 2001.
ArticleDouglas WW. Stimulus-secretion coupling: The concept and clues from chromaffin and other cells. Br J Pharmacol. 34:451–474. 1968.
ArticleDuarte J., Andriambeloson E., Diebolt M., Andriantsitohaina R. Wine polyphenols stimulate superoxide anion production to promote calcium signaling and endothelial-dependent vasodilatation. Physiol Res. 53:595–602. 2004.Fisher SK., Holz RW., Agranoff BW. Muscarinic receptors in chromaffin cell culture mediate enhanced phospholipid labeling but not catecholamine secretion. J Neurochem. 37:491–487. 1981.Fitzpatrick DF., Fleming RC., Bing B., Maggi DA., O'Malley R. Isolation and characterization of endothelium-dependent vaso-relaxing compounds from grape seeds. J Agric Food Chem. 48:6384–6390. 2000.
ArticleFitzpatrick DF., Hirschfield SL., Coffey RG. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am J Physiol. 265:H77–78. 1993.
ArticleFitzpatrick DF., Hirschfield SL., Ricci T., Jantzen P., Coffey RG. Endothelium-dependent vasorelaxation caused by various plant extracts. J Cardiovasc Pharmacol. 26:90–95. 1995.
ArticleFlesch M., Schwarz A., Bolun M. Effects of red and white wine on endothelium-dependent vasorelaxation of rat aorta and human coronary arteries. Am J Physiol. 275:H1183–H1190. 1998.
ArticleFrankel EN., Kanner J., German JB., Parks E., Kinsella JE. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet. 341:454–457. 1993a.
ArticleFrankel EN., Waterhouse AL., Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 341:1103–1104. 1993b.
ArticleFreedman JE., Li L., Sauter R., Keaney JF Jr. alpha-Tocopherol and protein kinase C inhibition enhance platelet-derived nitric oxide release. FASEB J. 14:2377–2379. 2000.Freedman NJ., Lefkowitz RJ. Anti-β1-adrenergic receptor antibodies and heart failure: causation, not just correlation. J Clin Invest. 113:1379–1382. 2004.Fuster V., Badimon JJ., Chesebro JH. The patogenesis of coronary artery disease and the acute coronary syndromes. New Engl J Med. 326:242–250. 1992.Garcia AG., Sala F., Reig JA., Viniegra S., Frias J., Fonteriz R., Gandia L. Dihydropyridine Bay-K-8644 activates chromaffin cell calcium channels. Nature. 309:69–71. 1984.
ArticleGerman JB., Walzem RL. The health benefits of wine. Annu Rev Nutr. 20:561–593. 2000.
ArticleGoeger DE., Riley RT. Interaction of cyclopiazonic acid with rat skeletal muscle sarcoplasmic reticulum vesicles. Effect on Ca2+ binding and Ca2+ permeability. Biochem Pharmacol. 38:3995–4003. 1989.Hammer R., Giachetti A. Muscarinic receptor subtypes: M1 and M2 biochemical and functional characterization. Life Sci. 31:2992–2998. 1982.Hashimoto M., Kim S., Eto M., Iijima K., Ako J., Yoshizumi M., Akishita M., Kondo K., Itakura H., Hosoda K., Toba K., Ouchi Y. Effect of acute intake of red wine on flow-mediated vasodilatation of the brachial artery. Am J Cardiol. 88:1457–1460. 2001.
ArticleHertog MGL., Kromhout D., Aravanis C., Blackburn H., Buzina R., Fidanza F., Giampaoli S., Jansen A., Menotti A., Nedeljkovic S. Flavonoid intake and long term risk of cardiovascular disease in the Seven Countries Study. Arch Intern Med. 155:381–386. 1995.Huang Y., Chan NWK., Lau CW., Yao XQ., Chan FL., Chen ZY. Involvement of endothelium/nilvicoxide in vasorelaxation induced by purified green tea (–) epicatechin. Biochim Biophys Acta. 1427:322–328. 1999.Huang Y., Zhang AQ., Lau CW., Chen ZY. Vasorelaxant effect of purified green tea epicatechin derivatives in rat mesenteric artery. Life Sci. 63:275–283. 1998.Ilno M. Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol. 94:363–383. 1989.Jendeková L., Kojsová S., Andriantsitohaina R., Pechánová O. The time-dependent effect of provinols on brain NO synthase activity in L-NAME-induced hypertension. Physiol Res. 55:S31–37. 2006.Kaye DM., Lefkowits J., Jennings GL., Bergin P., Broughton A., Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 26:1257–1263. 1995.
ArticleKidokoro Y., Ritchie AK. Chromaffin cell action potentials and their possible role in adrenaline secretion from rat adrenal medulla. J Physiol. 307:199–216. 1980.
ArticleKilpatrick DL., Slepetis RJ., Corcoran JJ., Kirshner N. Calcium uptake and catecholamine secretion by cultured bovine adrenal medulla cells. J Neurochem. 38:427–435. 1982.
ArticleKilpatrick DL., Slepetis RJ., Kirshner N. Ion channels and membrane potential in stimulus-secretion coupling in adrenal medulla cells. J Neurochem. 36:1245–1255. 1981.
ArticleKnight DE., Kesteven NT. Evoked transient intracellular free Ca2+ changes and secretion in isolated bovine adrenal medullary cells. Proc R Soc Lond Biol Sci. 218:177–199. 1983.Leikert JF., Räthel TR., Wohlfart P., Cheynier V., Vollmar AM., Dirsch VM. Red wine polyphenols enhance endothelial nitric oxide release from endothelial cells. Circulation. 106:1614–1617. 2002.Lim DY. Resveratrol inhibits catecholamine release from the adrenal medulla. J Hypertens. 26:S366. 2008.Lim DY., Hwang DH. Studies on secretion of catecholamines evoked by DMPP and McN-A-343 in the rat adrenal gland. Korean J Pharmacol. 27:53–67. 1991.Lim DY., Kim CD., Ahn KW. Influence of TMB-8 on secretion of catecholamines from the perfused rat adrenal glands. Arch Pharm Res. 15:115–125. 1992.
ArticleLiu S., Hu Y., Wang X., Zhong J., Lin Z. High content of resveratrol in lettuce transformed with a stilbene synthase gene of Parthenocissus henryana. J Agric Food Chem. 54:8082–8085. 2006.
ArticleLymperopoulos A., Rengo G., Funakoshi H., Eckhart AD., Koch WJ. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nat Med. 13:315–323. 2007.
ArticleMarley PD., McLeod J., Anderson C., Thomson KA. Nerves containing nitric oxide synthase and their possible function in the control of catecholamine secretion in the bovine adrenal medulla. J Auton Nerv Syst. 54:184–194. 1995.
ArticleMiddleton EJR., Kandaswami C., Theoharides TC. The effect of plant flavonoids on mammalian cells: Implications for inflammation, heart disease and cancer. Pharmacol Rev. 52:673–751. 2000.Mizutani K., Ikeda K., Kawai Y., Yamori Y. Extract of wine phenolics improves aortic biomechanical properties in stroke-prone spontaneously hypertensive rats (SHRSP). J Nutr Sci Vitaminol (Tokyo). 45:95–106. 1999.
ArticleOka M., Isosaki M., Yanagihara N. Isolated bovine adrenal medullary cells: studies on regulation of catecholamine synthesis and release. Usdin E, Kopin IJ, Brachas J, editors. ed,. Catecholamines: Basic and Clinical frontiers. Pergamon Press;Oxford: p. p. 70–72. 1979.
ArticleOset-Gasque MJ., Parramon M., Hortelano S., Bosca L., Gonzalez MP. Nitric oxide implication in the control of neurosecretion by chromaffin cells. J Neurochem. 63:1693–1700. 1994.
ArticleO'Sullivan AJ., Burgoyne RD. Cyclic GMP regulates nicotine-induced secretion from cultured bovine adrenal chromaffin cells: effects of 8-bromo-cyclic GMP, atrial natriuretic peptide, and nitroprusside (nitric oxide). J Neurochem. 54:1805–1808. 1990.Pace-Asciak CR., Hahn SE., Diamandis EP., Soleas G., Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implication for protection against coronary heart disease. Clin Chim Acta. 235:207–219. 1995.Palacios M., Knowles RG., Palmer RM., Moncada S. Nitric oxide from L-arginine stimulates the soluble guanylate cyclase in adrenal glands. Biochem Biophys Res Commun. 165:802–809. 1989.
ArticlePalmer RM., Ashton DS., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 333:664–666. 1988.
ArticlePechánová O., Bernátová I., Babál P., Martínez MC., Kyselá S., Stvrtina S., Andriantsitohaina R. Red wine polyphenols prevent cardiovascular alterations in L-NAME-induced hypertension. J Hypertens. 22:1551–1559. 2004.
ArticlePecháňová O., Zicha J., Kojšová S., Jendeková L., Sládková M., Paulis L., Janega P., Csizmádiová Z., Bernátová I., Dobešovš Z., šimko F., Babál P., Andriantsitohaina R., Kuneš J. Significance of antioxidants in experimental hypertension. Physiol Res. 55:3. 2006.Renaud S., de Lorgeril M. Wine alcohol, platelet and the French paradox for coronary heart disease. Lancet. 339:1523–1526. 1992.Rodriguez-Pascual F., Miras-Portugal MT., Torres M. Effect of cyclic GMP-increasing agents nitric oxide and C-type natriuretic peptide on bovine chromaffin cell function: inhibitory role mediated by cyclic GMP-dependent protein kinase. Mol Pharmacol. 49:1058–1070. 1996.Rotondo S., Rajtar G., Manarinis S. Effect of trans-resveratrol, a natural polyphenolic compound, on human polymorphonuclear leukocyte function. Br J Pharmacol. 123:1691–1699. 1998.
ArticleSakuma I., Stuehr DJ., Gross SS., Nathan C., Levi R. Identification of arginine as a precursor of endothelium-derived relaxing factor. Proc Natl Acad Sci USA. 85:8664–8667. 1988.
ArticleSchramm M., Thomas G., Towart R., Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 303:535–537. 1983.Schwarz PM., Rodriguez- Pascual F., Koesling D., Torres M., Förstermann U. Functional coupling of nitric oxide synthase and soluble guanylyl cyclase in controlling catecholamine secretion from bovine chromaffin cells. Neuroscience. 82:255–265. 1998.
ArticleSeidler NW., Jona I., Vegh N., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 264:17816–17823. 1989.Shimada K., Watanabe H., Hosoda K., Takeuchi K., Yoshikawa J. Effect of red wine on coronary flow-velocity reserve. Lancet. 354:1002. 1999.
ArticleSuzuki M., Muraki K., Imaizumi Y., Watanabe M. Cyclopiazonic acid, an inhibitor of the sarcoplasmic reticulum Ca2+-pump, reduces Ca2+-dependent K+ currents in guinea-pig smooth muscle cells. Br J Pharmacol. 107:134–140. 1992.Tallarida RJ., Murray RB. Manual of pharmacologic calculation with computer programs. 2nd ed.Speringer-Verlag;New York: p. p. 132. 1987.Torres M., Ceballos G., Rubio R. Possible role of nitric oxide in catecholamine secretion by chromaffin cells in the presence and absence of cultured endothelial cells. J Neurochem. 63:988–996. 1994.
ArticleUchiyama Y., Morita K., Kitayama S., Suemitsu T., Minami N., Miyasako T., Dohi T. Possible involvement of nitric oxide in acetylcholine-induced increase of intracellular Ca2+ concentration and catecholamine release in bovine adrenal chromaffin cells. Jpn J Pharmacol. 65:73–77. 1994.Uyama Y., Imaizumi Y., Watanabe M. Effects of cyclopiazonic acid, a novel Ca2+-ATPase inhibitor on contractile responses in skinned ileal smooth muscle. Br J Pharmacol. 106:208–214. 1992.Wada Y., Satoh K., Taira N. Cardiovascular profile of Bay-K-8644, a presumed calcium channel activator in the dog. Naunyn-Schmiedebergs Arch Pharmacol. 328:382–387. 1985a.Wada A., Takara H., Izumi F., Kobayashi H., Yanagihara N. Influx of 22Na through acetylcholine receptor-associated Na channels: relationship between 22Na influx, 45Ca influx and secretion of catecholamines in cultured bovine adrenal medullary cells. Neuroscience. 15:283–292. 1985b.Wakade AR. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 313:463–480. 1981.
ArticleWakade AR., Wakade TD. Contribution of nicotinic and muscarinic receptors in the secretion of catecholamines evoked by endogenous and exogenous acetylcholine. Neuroscience. 10:973–978. 1983.
ArticleWallerath T., Deckert G., Ternes T., Anderson H., Li H., Witte K., Förstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 106:1652–1658. 2002.
ArticleWestfall TC., Westfall DP. Adrenergic agonists and antagonists. Brunton LL, Lazo JS, Parker KL, editors. ed,. Goodman & Gilman the pharmacological basis of therapeutics. 11th ed.McGraw-Hill;New York: p. p. 237–295. 2005.Yanagihara N., Isosaki M., Ohuchi T., Oka M. Muscarinic receptor-mediated increase in cyclic GMP level in isolated bovine adrenal medullary cells. FEBS Lett. 105:296–298. 1979.
ArticleZenebe W., Pecháóová O. Effects of red wine polyphenolic compounds on the cardiovascular system. Bratisl Lek Listy. 103:159–165. 2002.Zenebe W., Pecháóová O., Andriantsitohaina R. Red wine polyphenols induce vasorelaxation by increased nitric oxide bioactivity. Physiol Res. 52:425–432. 2003.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of SKF81297 on Catecholamine Release from the Perfused Rat Adrenal Medulla
- Effects of Losartan on Catecholamine Release in the Isolated Rat Adrenal Gland
- Inhibitory Effects of Olmesartan on Catecholamine Secretion from the Perfused Rat Adrenal Medulla
- R-(-)-TNPA, a Dopaminergic D2 Receptor Agonist, Inhibits Catecholamine Release from the Rat Adrenal Medulla
- D-Amphetamine Causes Dual Actions on Catecholamine Release from the Rat Adrenal Medulla