Korean J Urol.
2014 Dec;55(12):834-840. 10.4111/kju.2014.55.12.834.
Tumor-Specific Immunity Induced by Cryoablation in a Murine Renal Cell Carcinoma Model
- Affiliations
-
- 1Department of Urology, Korea University Anam Hospital, Seoul, Korea. mdksh@korea.ac.kr
- 2Department of Urology, Korea University Guro Hospital, Seoul, Korea.
- KMID: 2070099
- DOI: http://doi.org/10.4111/kju.2014.55.12.834
Abstract
- PURPOSE
To evaluate tumor-specific immunity and define the mechanisms involved in the cryoimmunologic response, we compared the tumor control efficacy and immunologic responses of cryoablation with those of surgical excision in a tumor rechallenge model.
MATERIALS AND METHODS
Sixty BALB/c mice with RENCA tumors that were generated in the left flank area underwent cryoablation or radical excision. The mice successfully treated were rechallenged with RENCA or an undifferentiated colon carcinoma cell line, CT26, in the contralateral right flank area. The recurrence rate after tumor rechallenge in each group was then observed. To assess the immunologic response of each treatment modality, fluorescent-activated cell sorting (FACS) analysis and a cytotoxicity assay using 51Cr release were performed.
RESULTS
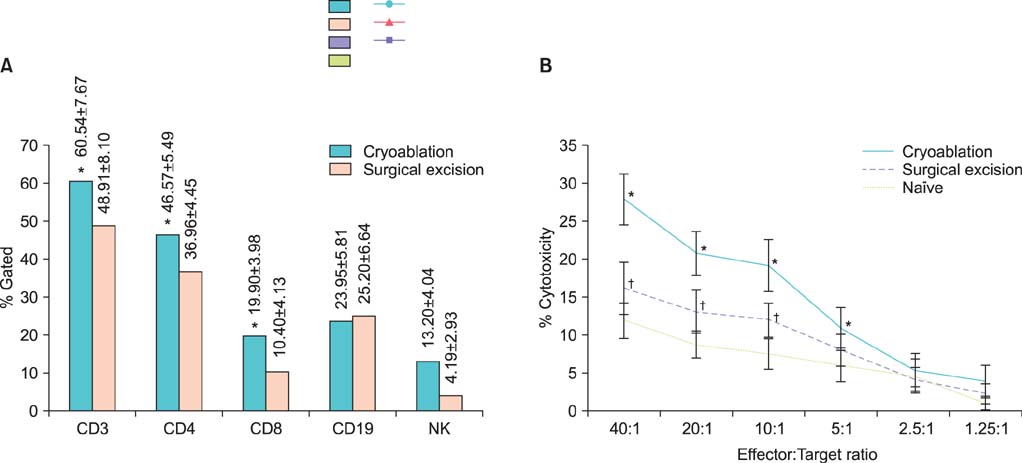
After reinoculation of the RENCA cells, the rate of tumor growth was significantly higher in the surgical excision group than in the cryoablation group (94.4% vs. 11.1%, p=0.001). In the cryoablation group, the tumor growth rate was significantly increased after rechallenge of CT26 cells compared with RENCA (94.1% vs. 11.1%, p=0.001). The cryoablation group showed an elevated CD3, CD4, CD8 T, and natural killer cell count in the FACS analysis and also showed significantly increased cytotoxicity in the 51Cr release assay compared with the excision group.
CONCLUSIONS
These results showed that cryoablation, compared to surgical resection, was more effective in preventing tumor growth after rechallenge with RENCA cells and that this response was tumor-specific, because the CT26 cells did not have the same effect.
Keyword
MeSH Terms
-
Animals
CD4-Positive T-Lymphocytes/immunology
CD8-Positive T-Lymphocytes/immunology
Carcinoma, Renal Cell/*immunology/pathology/*surgery
Cell Death
Cryosurgery/*methods
Cytotoxicity, Immunologic
Disease Models, Animal
Kidney Neoplasms/*immunology/pathology/*surgery
Lymphocyte Count
Lymphocytes, Tumor-Infiltrating/immunology
Mice, Inbred BALB C
Neoplasm Recurrence, Local/immunology
Neoplasm Transplantation
Figure
Reference
-
1. deKernion JB. Treatment of advanced renal cell carcinoma: traditional methods and innovative approaches. J Urol. 1983; 130:2–7.2. Rabinovitch RA, Zelefsky MJ, Gaynor JJ, Fuks Z. Patterns of failure following surgical resection of renal cell carcinoma: implications for adjuvant local and systemic therapy. J Clin Oncol. 1994; 12:206–212.3. Skinner DG, Colvin RB, Vermillion CD, Pfister RC, Leadbetter WF. Diagnosis and management of renal cell carcinoma. A clinical and pathologic study of 309 cases. Cancer. 1971; 28:1165–1177.4. Oosterwijk E, Divgi CR, Brouwers A, Boerman OC, Larson SM, Mulders P, et al. Monoclonal antibody-based therapy for renal cell carcinoma. Urol Clin North Am. 2003; 30:623–631.5. Jacobsohn KM, Wood CG. Adjuvant therapy for renal cell carcinoma. Semin Oncol. 2006; 33:576–582.6. Mabjeesh NJ, Avidor Y, Matzkin H. Emerging nephron sparing treatments for kidney tumors: a continuum of modalities from energy ablation to laparoscopic partial nephrectomy. J Urol. 2004; 171(2 Pt 1):553–560.7. Hoffmann NE, Bischof JC. The cryobiology of cryosurgical injury. Urology. 2002; 60:2 Suppl 1. 40–49.8. Sidana A, Rodriguez R. Urologic applications of cryo-immunology. Korean J Urol. 2009; 50:629–634.9. Johnson JP. Immunologic aspects of cryosurgery: potential modulation of immune recognition and effector cell maturation. Clin Dermatol. 1990; 8:39–47.10. Gage AA. Cryosurgery for oral and pharyngeal carcinoma. Am J Surg. 1969; 118:669–672.11. Soanes WA, Ablin RJ, Gonder MJ. Remission of metastatic lesions following cryosurgery in prostatic cancer: immunologic considerations. J Urol. 1970; 104:154–159.12. Neel HB 3rd, Ritts RE Jr. Immunotherapeutic effect of tumor necrosis after cryosurgery, electrocoagulation, and ligation. J Surg Oncol. 1979; 11:45–52.13. Lubaroff DM, Reynolds CW, Canfield L, McElligott D, Feldbush T. Immunologic aspects of the prostate. Prostate. 1981; 2:233–248.14. Sabel MS, Nehs MA, Su G, Lowler KP, Ferrara JL, Chang AE. Immunologic response to cryoablation of breast cancer. Breast Cancer Res Treat. 2005; 90:97–104.15. Ablin RJ, Soanes WA, Gonder MJ. Elution of in vivo bound antiprostatic epithelial antibodies following multiple cryotherapy of carcinoma of prostate. Urology. 1973; 2:276–279.16. Eskandari H, Ablin RJ, Bhatti RA. Immunologic responsiveness & tumour growth of the Dunning R3327 rat prostatic adenocarcinoma following cryosurgery & orchiectomy. Indian J Exp Biol. 1982; 20:872–874.17. Playfair JH, Chain BM. Immunity to tumours. In : Playfair JH, Chain BM, editors. Immunology at a glance. 8th ed. Malden: Blackwell;2005. p. 70–71.18. Abbas AK, Lichtman AH. Cellular and molecular immunology. 5th ed. Singapore: Elsevier;2004.19. Aron M, Gill IS. Minimally invasive nephron-sparing surgery (MINSS) for renal tumours. Part II: probe ablative therapy. Eur Urol. 2007; 51:348–357.20. Onik GM, Reyes G, Cohen JK, Porterfield B. Ultrasound characteristics of renal cryosurgery. Urology. 1993; 42:212–215.21. Matin SF, Sharma P, Gill IS, Tannenbaum C, Hobart MG, Novick AC, et al. Immunological response to renal cryoablation in an in vivo orthotopic renal cell carcinoma murine model. J Urol. 2010; 183:333–338.22. Osada S, Imai H, Tomita H, Tokuyama Y, Okumura N, Matsuhashi N, et al. Serum cytokine levels in response to hepatic cryoablation. J Surg Oncol. 2007; 95:491–498.23. Udagawa M, Kudo-Saito C, Hasegawa G, Yano K, Yamamoto A, Yaguchi M, et al. Enhancement of immunologic tumor regression by intratumoral administration of dendritic cells in combination with cryoablative tumor pretreatment and Bacillus Calmette-Guerin cell wall skeleton stimulation. Clin Cancer Res. 2006; 12:7465–7475.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-tumor Immune Response after Cryoablation in Renal Cell Carcinoma Murine Model
- Immunologic Response to Cryoablation of Squamous Cell Carcinoma
- Antitumor Effect of in Situ Cryoablation with Systemic Immunotherapy on Murine Renal Cell Tumor
- Transient urine leakage following cryoablation: case report
- Dendritic Cell Based Cancer Immunotherapy: in vivo Study with Mouse Renal Cell Carcinoma Model