Korean J Urol.
2008 Nov;49(11):965-973. 10.4111/kju.2008.49.11.965.
Antitumor Effect of in Situ Cryoablation with Systemic Immunotherapy on Murine Renal Cell Tumor
- Affiliations
-
- 1Department of Urology, College of Medicine, Korea University, Seoul, Korea. dkyoon@korea.ac.kr
- KMID: 1328166
- DOI: http://doi.org/10.4111/kju.2008.49.11.965
Abstract
-
PURPOSE: To investigate synergistic effect of local cryoablation with systemic immunotherapy, the tumor control ability and immunologic responses of combining these two modalities was compared with that of cryoablation, surgical excision, and immunotherapy only group in a tumor re-challenge model.
MATERIALS AND METHODS
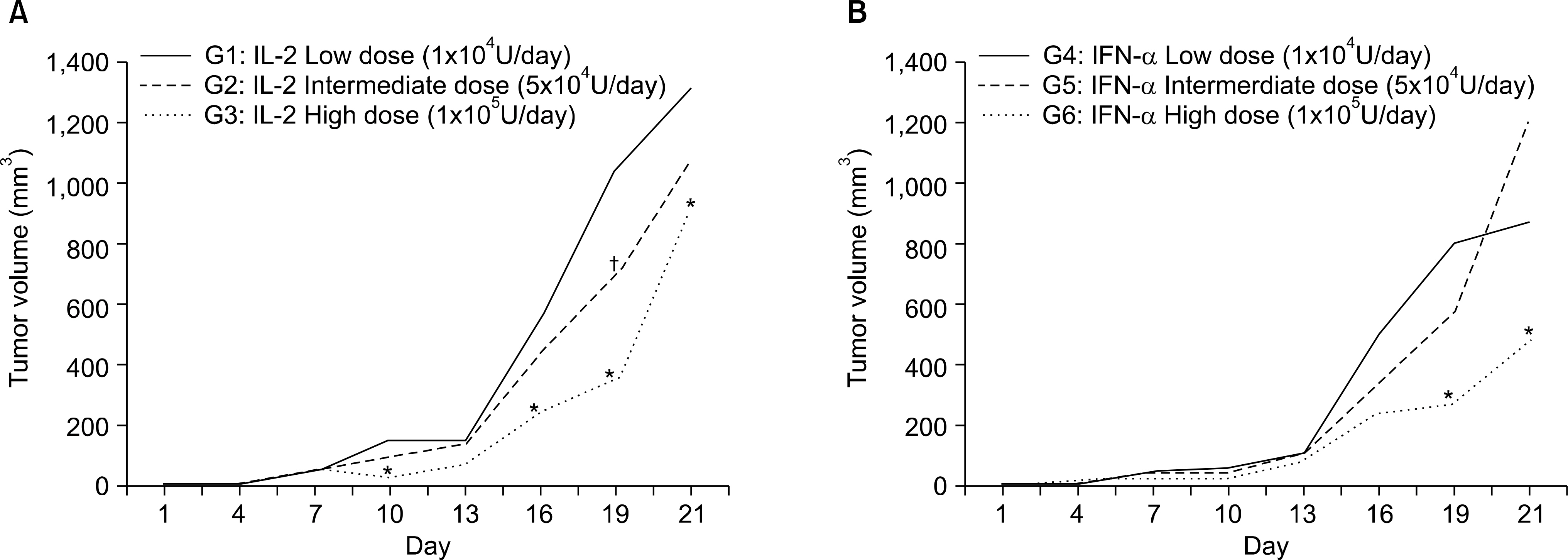
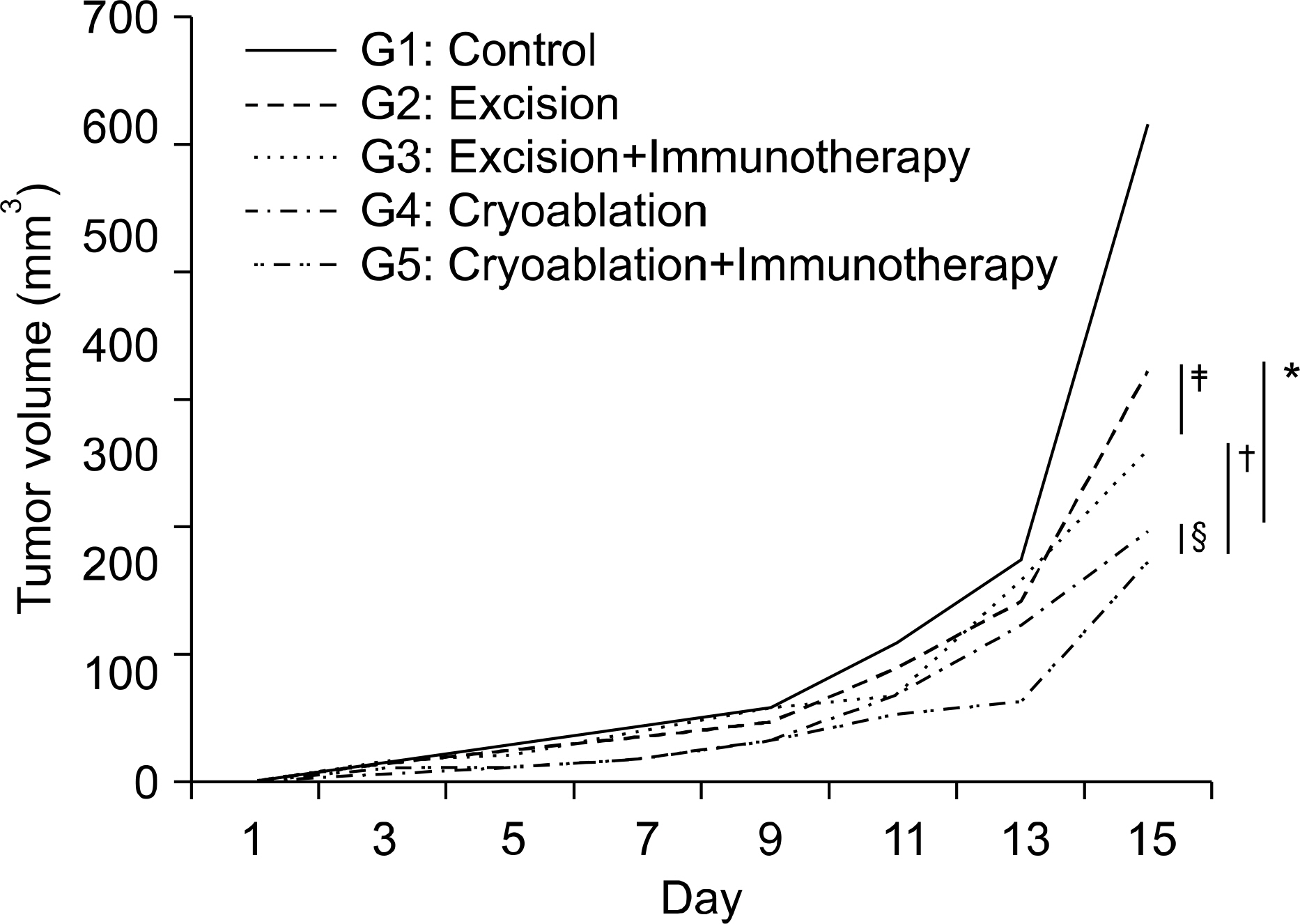
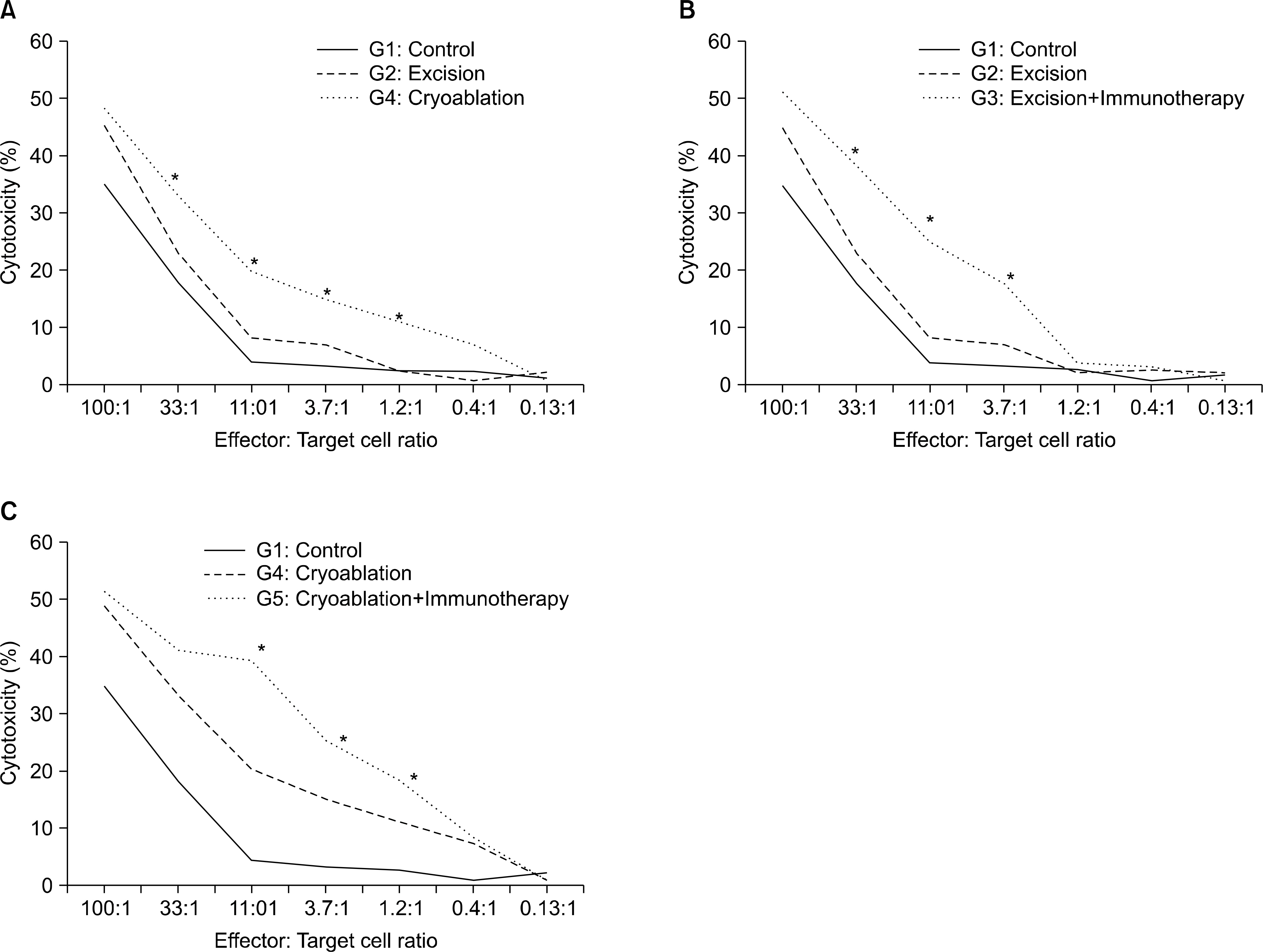
Preliminary experiments were performed in two stages. The first stage consisted of 36 Balb/c mice with Renca bearing tumors imbedded in the right thigh, and was treated with interleukin-2 (IL-2) and interferon-alpha(IFN-alpha) to evaluate the efficacy of immunotherapy and to determine the adequate dosage. The second stage was performed on 10 mice, to evaluate histological changes and efficacy after cryoablation. The main experiment was performed on 48 mice, divided into 6 groups of control with tumor implantation, excision of tumor, excision combined with immunotherapy, cryoablation of tumor, cryoablation with immunotherapy and control without tumor. After treatment, tumor re-challenge was performed with Renca cell, then the growth pattern was evaluated with physical measurements, and immune response was investigated with fluorescent activated cell sorter and cytotoxicity assay.
RESULTS
Preliminary studies on immunologic efficacy revealed that IL-2 and IFN-alpha have a dose dependent inhibition of tumor growth. The main experiment evaluating the efficacy of combination treatment revealed that cryoablation with immunotherapy proved to be most effective in terms of tumor recurrence and tumor growth inhibition, yet the difference was not statistically significant from monotherapy with cryoablation. However, cytotoxicity was significantly increased cryoablation with immunotherapy compared with other groups.
CONCLUSIONS
Cryoablation on tumor re-challenge mice model showed advantages with immunotherapy most prominently in cytotoxicity.
Keyword
Figure
Reference
-
References
1. Flanigan RC. Debulking nephrectomy in metastatic renal cancer. Clin Cancer Res. 2004; 10:S6335–41.
Article2. Jacobs SC, Berg SI, Lawson RK. Synchronous bilateral renal cell carcinoma: total surgical excision. Cancer. 1980; 46:2341–5.
Article3. Moll V, Becht E, Ziegler M. Kidney preserving surgery in renal cell tumors: indications, techniques and results in 152 patients. J Urol. 1993; 150:319–23.
Article4. Belldegrun A, Tsui KH, deKernion JB, Smith RB. Efficacy of nephron-sparing surgery for renal cell carcinoma: analysis based on the new 1997 tumor-node-metastasis staging system. J Clin Oncol. 1999; 17:2868–75.
Article5. Patard JJ, Kim HL, Lam JS, Dorey FJ, Pantuck AJ, Zisman A, et al. Use of the University of California Los Angeles integrated staging system to predict survival in renal call carcinoma: an international multicenter study. J Clin Oncol. 2004; 22:3316–22.6. Campbell SC, Novick AC, Bukowski RM. Renal tumors. Kavoussi LR, Novick AC, Partin AW, Peters CA, Wein AJ, editors. editors.Campbell's urology. 9th ed.Philadelphia: Saunders;2007. p. 1567–637.7. Colimbu M, Joshi P, Sperber A, Tessler A, Al-Askari S, Morales P. Renal cell carcinoma: survival and prognostic factors. Urology. 1986; 27:291–301.
Article8. Hafez KS, Montie JE. Indication and limitations of cytoreductive nephrectomy for metastatic renal cell carcinoma. AUA Update Series. 2004. ;lesson 31.9. O'Malley RL, Berger AD, Kanofsky JA, Phillips CK, Stifel-man M, Taneja SS. A matched-cohort comparison of laparoscopic cryoablation and laparoscopic partial nephrectomy for treating renal masses. BJU Int. 2007; 99:395–8.10. Hruby G, Reisiger K, Venkatesh R, Yan Y, Landman J. Comparison of laparoscopic partial nephrectomy and laparoscopic cryoablation for renal hilar tumors. Urology. 2006; 67:50–4.
Article11. Ablin RJ. An appreciation and realization of the concept of cryoimmunology. Ablin RJ, editor. Percutaneous prostate cryoablation. 1st ed.St. Louis: Quality Medical Publishing;1995. p. 136–54.12. Suzuki Y. Cryosurgical treatment of advanced breast cancer and cryoimmunological responses. Skin Cancer. 1995; 10:19–26.13. Hornung RL, Back TC, Zaharko DS, Urba WJ, Longo DL, Wiltrout RH. Augmentation of natural killer activity, induction of IFN and development tumor immunity during the successful treatment of established murine renal cancer using flavone acetic acid and IL-2. J Immunol. 1988; 141:3671–9.14. Sayers TJ, Wiltrout TA, McCormick K, Husted C, Wiltrout RH. Antitumor effects of alpha-interferon and gamma-interferon on a murine renal cancer (Renca) in vitro and in vivo. Cancer Res. 1990; 50:5414–20.15. Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year follow up. J Urol. 2000; 163:442–5.16. Gage AA, Huben R. Cryosurgical ablation of the prostate. Urol Oncol. 2000; 5:19–24.
Article17. Sabel MS, Edge SB. In-situ ablation of breast cancer. Breast Dis. 2001; 12:131–40.
Article18. Johnson JP. Immunologic aspects of cryosurgery: potential modulation of immune recognition and effector cell maturation. Clin Dermatol. 1990; 8:39–47.
Article19. Sabel M, Arora A, Su G, Chang AE. Adoptive immunotherapy of breat cancer with lymph node cells primed by cryoablation of the primary tumor. Cryobiology. 2006; 53:360–6.20. Ablin RJ, Jagodzinski RV, Prox C, Williams RW, Gonder MJ, Soanes WA. Cryosurgery of the monkey (macaque) prostate. I. Humoral immunologic responsiveness following cryosti-mulation. Cryobiology. 1976; 13:47–53.21. Airoldi M, Fazio M, Gandolfo S, Ozzello F, Pedani F, Brando V, et al. Combined chemotherapy, cryosurgery, and radiotherapy/surgery for oral cancer. Int J Clin Pharmacol Res. 1985; 5:357–62.22. Fujikawa S, Suzuki T, Ishikawa T, Sakurai S, Hasegawa Y. Continuous observation of frozen biological materials with cryo-scanning electron microscope and freeze-replica by a new cryo-system. J Electron Microsc. 1988; 37:315–22.23. Kang SH, Bae JH, Shim KS. The development of tumour-specific immunity induced by cryosurgery in murine renal cell carcinoma animal model. Eur Urol. 2006; 5(Suppl):): 29.
Article24. Kaouk JH, Aron M, Rewcastle JC, Gill IS. Cryotherapy: clinical end points and their experimental foundations. Urology. 2006; 68(1 Suppl):38–44.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-tumor Immune Response after Cryoablation in Renal Cell Carcinoma Murine Model
- Effect of BCG Immunotherapy on the Cytokine Production and Antitumor Activity against MBT - 2 Mouse Bladder Tumor
- Immunologic Response to Cryoablation of Squamous Cell Carcinoma
- Urologic Applications of Cryo-Immunology
- The Initial Experience with 3rd Generation Nephron-sparing Cryoablation for Renal Tumor