Korean J Urol.
2012 Sep;53(9):636-642. 10.4111/kju.2012.53.9.636.
Differential Expression of Nerve Injury-Induced Protein 1 (Ninjurin 1) in In Vivo and In Vitro Models for Diabetic Erectile Dysfunction
- Affiliations
-
- 1Department of Urology, National Research Center for Sexual Medicine, Inha University School of Medicine, Incheon, Korea. jksuh@inha.ac.kr
- KMID: 2061703
- DOI: http://doi.org/10.4111/kju.2012.53.9.636
Abstract
- PURPOSE
Endothelial dysfunction and peripheral neuropathy are important mechanisms responsible for diabetes-induced erectile dysfunction (ED). Nerve injury-induced protein 1 (Ninjurin 1) is known to be related to neuroinflammatory processes and is also reported to induce vascular regression during the developmental period. In the present study, we determined the differential expression of Ninjurin 1 in penile tissue of streptozotocin (STZ)-induced diabetic mice with ED.
MATERIALS AND METHODS
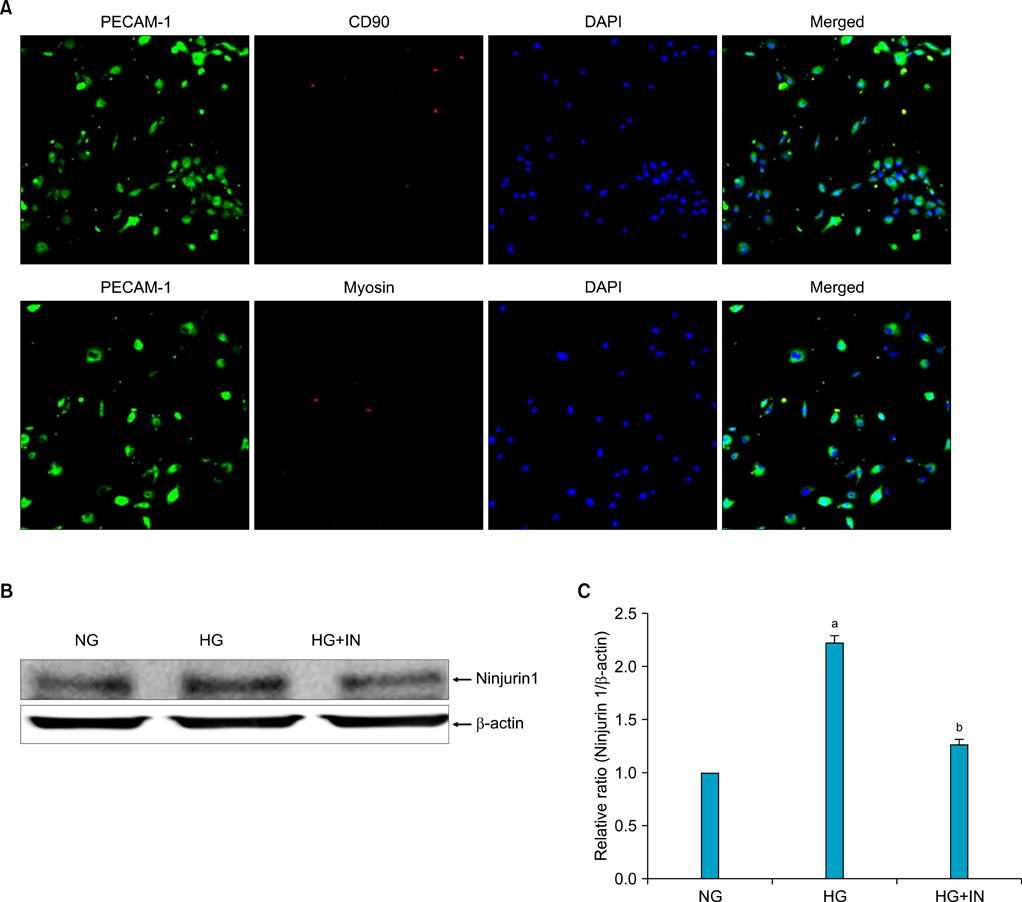
Diabetes was induced in 8-week-old C57BL/6J mice by intraperitoneal injections of STZ (50 mg/kg for 5 days). Eight weeks later, erectile function was measured by electrical stimulation of the cavernous nerve (n=6 per group). The penis was then harvested for immunohistochemical analysis and Western blot analysis for Ninjurin 1 (n=4 per group). We also determined Ninjurin 1 expression in primary cultured mouse cavernous endothelial cells (MCECs) incubated under the following conditions: normal glucose condition (5 mM), high-glucose condition (30 mM), and high-glucose condition (30 mM)+insulin (1 nM).
RESULTS
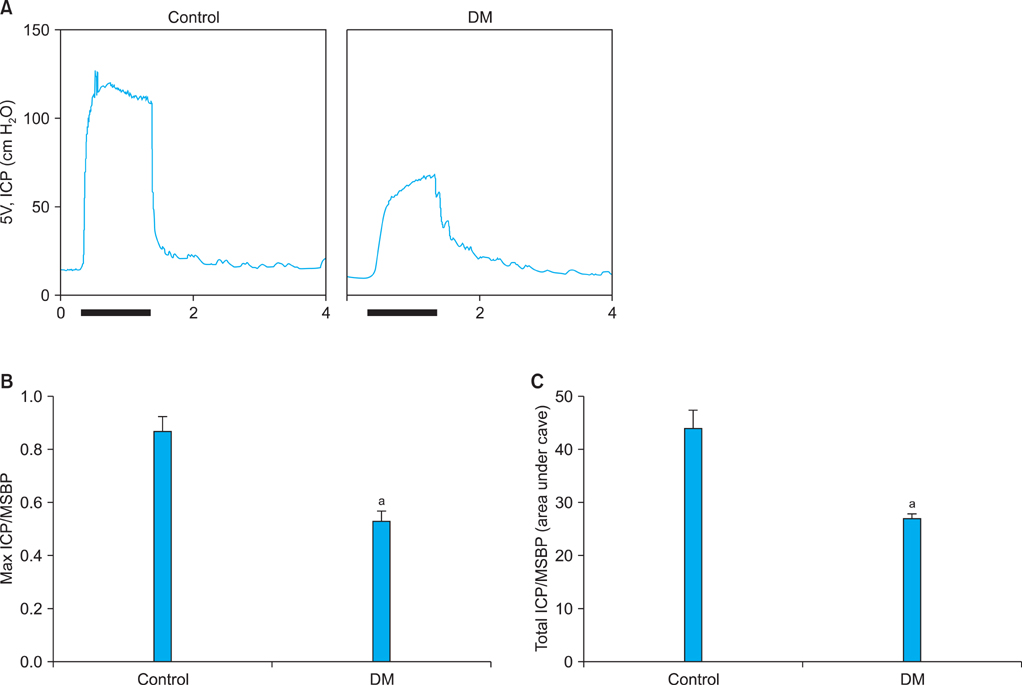
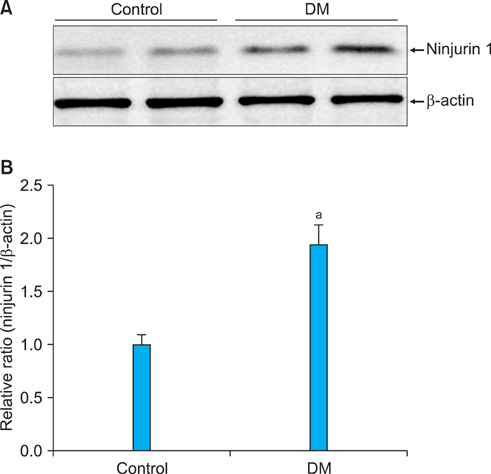
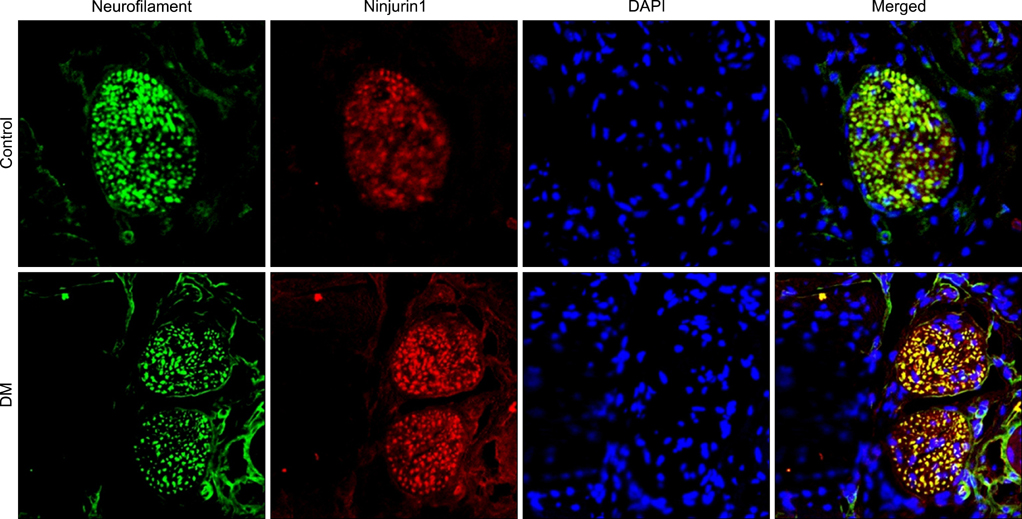
The expression of Ninjurin 1 protein was significantly higher in both cavernous endothelial cells and the dorsal nerve bundle of diabetic mice than in those of controls. In the in vitro study in MCECs, Ninjurin 1 expression was also significantly increased by the high-glucose condition and was returned to baseline levels by treatment with insulin.
CONCLUSIONS
Regarding the role of Ninjurin 1 in neuropathy and vascular regression, it would be interesting to examine the effects of inhibition of Ninjurin 1 on erectile function in animal models of ED with a vascular or neurogenic cause.
Keyword
MeSH Terms
Figure
Reference
-
1. Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med. 2009. 6:1232–1247.2. Hakim LS, Goldstein I. Diabetic sexual dysfunction. Endocrinol Metab Clin North Am. 1996. 25:379–400.3. Burchardt T, Burchardt M, Karden J, Buttyan R, Shabsigh A, de la Taille A, et al. Reduction of endothelial and smooth muscle density in the corpora cavernosa of the streptozotocin induced diabetic rat. J Urol. 2000. 164:1807–1811.4. Ahn GJ, Sohn YS, Kang KK, Ahn BO, Kwon JW, Kang SK, et al. The effect of PDE5 inhibition on the erectile function in streptozotocin-induced diabetic rats. Int J Impot Res. 2005. 17:134–141.5. Jin HR, Kim WJ, Song JS, Choi MJ, Piao S, Shin SH, et al. Functional and morphologic characterizations of the diabetic mouse corpus cavernosum: comparison of a multiple low-dose and a single high-dose streptozotocin protocols. J Sex Med. 2009. 6:3289–3304.6. El-Sakka AI, Lin CS, Chui RM, Dahiya R, Lue TF. Effects of diabetes on nitric oxide synthase and growth factor genes and protein expression in an animal model. Int J Impot Res. 1999. 11:123–132.7. Zhang XH, Filippi S, Morelli A, Vignozzi L, Luconi M, Donati S, et al. Testosterone restores diabetes-induced erectile dysfunction and sildenafil responsiveness in two distinct animal models of chemical diabetes. J Sex Med. 2006. 3:253–264.8. Rendell MS, Rajfer J, Wicker PA, Smith MD. Sildenafil Diabetes Study Group. Sildenafil for treatment of erectile dysfunction in men with diabetes: a randomized controlled trial. JAMA. 1999. 281:421–426.9. Martínez-Jabaloyas JM, Gil-Salom M, Villamon-Fort R, Pastor-Hernandez F, Martinez-Garcia R, Garcia-Sisamon F. Prognostic factors for response to sildenafil in patients with erectile dysfunction. Eur Urol. 2001. 40:641–646.10. Musicki B, Burnett AL. Endothelial dysfunction in diabetic erectile dysfunction. Int J Impot Res. 2007. 19:129–138.11. De Young L, Yu D, Bateman RM, Brock GB. Oxidative stress and antioxidant therapy: their impact in diabetes-associated erectile dysfunction. J Androl. 2004. 25:830–836.12. Araki T, Milbrandt J. Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron. 1996. 17:353–361.13. Ifergan I, Kebir H, Terouz S, Alvarez JI, Lecuyer MA, Gendron S, et al. Role of Ninjurin-1 in the migration of myeloid cells to central nervous system inflammatory lesions. Ann Neurol. 2011. 70:751–763.14. Lee HJ, Ahn BJ, Shin MW, Jeong JW, Kim JH, Kim KW. Ninjurin1 mediates macrophage-induced programmed cell death during early ocular development. Cell Death Differ. 2009. 16:1395–1407.15. Yin GN, Ryu JK, Kwon MH, Shin SH, Jin HR, Song KM, et al. Matrigel-based sprouting endothelial cell culture system from mouse corpus cavernosum is potentially useful for the study of endothelial and erectile dysfunction related to high-glucose exposure. J Sex Med. 2012. 9:1760–1772.16. Saenz de Tejada I, Goldstein I, Azadzoi K, Krane RJ, Cohen RA. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989. 320:1025–1030.17. Cellek S, Rodrigo J, Lobos E, Fernández P, Serrano J, Moncada S. Selective nitrergic neurodegeneration in diabetes mellitus : a nitric oxide-dependent phenomenon. Br J Pharmacol. 1999. 128:1804–1812.18. McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007. 8:913–919.19. Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009. 119:182–192.20. Walsh PC, Mostwin JL. Radical prostatectomy and cystoprostatectomy with preservation of potency. Results using a new nerve-sparing technique. Br J Urol. 1984. 56:694–697.21. Noldus J, Michl U, Graefen M, Haese A, Hammerer P, Huland H. Patient-reported sexual function after nerve-sparing radical retropubic prostatectomy. Eur Urol. 2002. 42:118–124.22. Catalona WJ, Bigg SW. Nerve-sparing radical prostatectomy: evaluation of results after 250 patients. J Urol. 1990. 143:538–543.23. Catalona WJ, Basler JW. Return of erections and urinary continence following nerve sparing radical retropubic prostatectomy. J Urol. 1993. 150:905–907.24. Kim SW. Prostatic disease and sexual dysfunction. Korean J Urol. 2011. 52:373–378.25. Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009. 10:165–177.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical localization of nerve injury-induced protein-1 in mouse tissues
- Comparison of Erectile Response to Intracavernous Injection Therapy in Diabetic and Non-diabetic Erectile Dysfunction Patients
- Inhibition of MicroRNA-92a Improved Erectile Dysfunction in Streptozotocin-Induced Diabetic Rats via Suppressing Oxidative Stress and Endothelial Dysfunction
- Pericyte-derived heme-binding protein 1 promotes angiogenesis and improves erectile function in diabetic mice
- Induction of Nerve Injury-Induced Protein 1 (Ninjurin 1) in Myeloid Cells in Rat Brain after Transient Focal Cerebral Ischemia