J Periodontal Implant Sci.
2012 Apr;42(2):50-58. 10.5051/jpis.2012.42.2.50.
Periodontal tissue reaction to customized nano-hydroxyapatite block scaffold in one-wall intrabony defect: a histologic study in dogs
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. shchoi726@yuhs.ac
- 2Department of Dental Biomaterials and Bioengineering, Research Institute of Dental Biomaterials and Bioengineering, Yonsei University College of Dentistry, Seoul, Korea.
- KMID: 2027787
- DOI: http://doi.org/10.5051/jpis.2012.42.2.50
Abstract
- PURPOSE
This study evaluated histologically the tissue responses to and the effects of a customized nano-hydroxyapatite (n-HA) block bone graft on periodontal regeneration in a one-wall periodontal-defect model.
METHODS
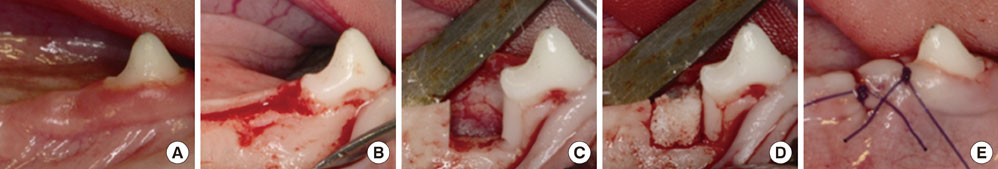
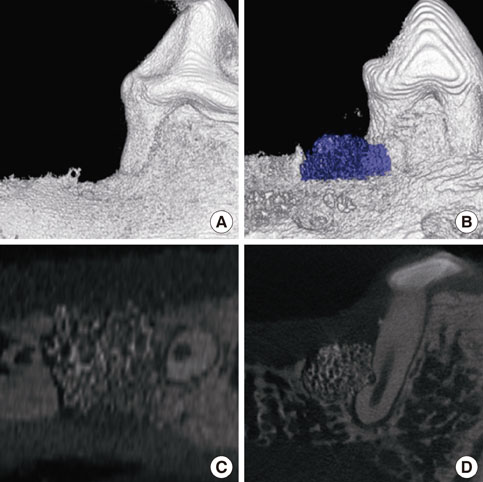
A customized block bone for filling in the standardized periodontal defect was fabricated from prefabricated n-HA powders and a polymeric sponge. Bilateral 4x4x5 mm (buccolingual widthxmesiodistal widthxdepth), one-wall, critical-size intrabony periodontal defects were surgically created at the mandibular second and fourth premolars of five Beagle dogs. In each dog, one defect was filled with block-type HA and the other served as a sham-surgery control. The animals were sacrificed following an 8-week healing interval for clinical and histological evaluations.
RESULTS
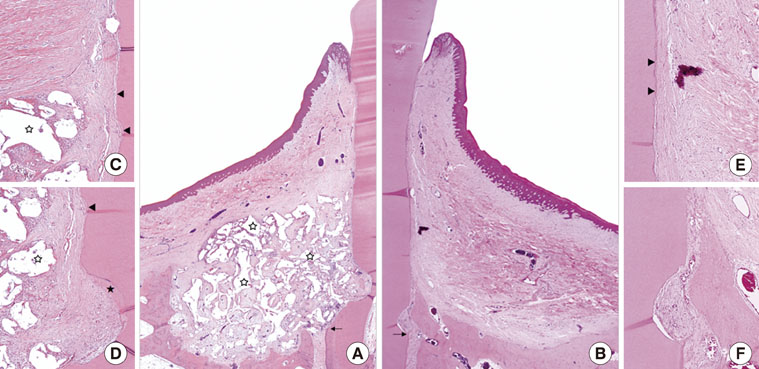
Although the sites that received an n-HA block showed minimal bone formation, the n-HA block was maintained within the defect with its original hexahedral shape. In addition, only a limited inflammatory reaction was observed at sites that received an n-HA block, which might have been due to the high stability of the customized block bone.
CONCLUSIONS
In the limitation of this study, customized n-HA block could provide a space for periodontal tissue engineering, with minimal inflammation.
Keyword
MeSH Terms
Figure
Reference
-
1. Bartold PM, McCulloch CA, Narayanan AS, Pitaru S. Tissue engineering: a new paradigm for periodontal regeneration based on molecular and cell biology. Periodontol 2000. 2000. 24:253–269.
Article2. Trombelli L. Which reconstructive procedures are effective for treating the periodontal intraosseous defect? Periodontol 2000. 2005. 37:88–105.
Article3. Bender SA, Rogalski JB, Mills MP, Arnold RM, Cochran DL, Mellonig JT. Evaluation of demineralized bone matrix paste and putty in periodontal intraosseous defects. J Periodontol. 2005. 76:768–777.
Article4. Caton J, Nyman S, Zander H. Histometric evaluation of periodontal surgery. II. Connective tissue attachment levels after four regenerative procedures. J Clin Periodontol. 1980. 7:224–231.
Article5. Heitz-Mayfield L, Tonetti MS, Cortellini P, Lang NP. European Research Group on Periodontology (ERGOPERIO). Microbial colonization patterns predict the outcomes of surgical treatment of intrabony defects. J Clin Periodontol. 2006. 33:62–68.
Article6. Machtei EE, Cho MI, Dunford R, Norderyd J, Zambon JJ, Genco RJ. Clinical, microbiological, and histological factors which influence the success of regenerative periodontal therapy. J Periodontol. 1994. 65:154–161.
Article7. Schulz A, Hilgers RD, Niedermeier W. The effect of splinting of teeth in combination with reconstructive periodontal surgery in humans. Clin Oral Investig. 2000. 4:98–105.
Article8. Needleman IG, Worthington HV, Giedrys-Leeper E, Tucker RJ. Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database Syst Rev. 2006. (2):CD001724.
Article9. Ciocca L, De Crescenzio F, Fantini M, Scotti R. CAD/CAM and rapid prototyped scaffold construction for bone regenerative medicine and surgical transfer of virtual planning: a pilot study. Comput Med Imaging Graph. 2009. 33:58–62.
Article10. Dellinger JG, Cesarano J 3rd, Jamison RD. Robotic deposition of model hydroxyapatite scaffolds with multiple architectures and multiscale porosity for bone tissue engineering. J Biomed Mater Res A. 2007. 82:383–394.
Article11. Sun W, Darling A, Starly B, Nam J. Computer-aided tissue engineering: overview, scope and challenges. Biotechnol Appl Biochem. 2004. 39(Pt 1):29–47.
Article12. Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000. 21:2529–2543.
Article13. Benlidayi ME, Kurkcu M, Oz IA, Sertdemir Y. Comparison of two different forms of bovine-derived hydroxyapatite in sinus augmentation and simultaneous implant placement: an experimental study. Int J Oral Maxillofac Implants. 2009. 24:704–711.
Article14. De Santis E, Botticelli D, Pantani F, Pereira FP, Beolchini M, Lang NP. Bone regeneration at implants placed into extraction sockets of maxillary incisors in dogs. Clin Oral Implants Res. 2011. 22:430–437.
Article15. Jensen T, Schou S, Stavropoulos A, Terheyden H, Holmstrup P. Maxillary sinus floor augmentation with Bio-Oss or Bio-Oss mixed with autogenous bone as graft: a systematic review. Clin Oral Implants Res. 2012. 23:263–273.
Article16. Wang H, Li Y, Zuo Y, Li J, Ma S, Cheng L. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials. 2007. 28:3338–3348.
Article17. de Bruijn JD, van Blitterswijk CA, Davies JE. Initial bone matrix formation at the hydroxyapatite interface in vivo. J Biomed Mater Res. 1995. 29:89–99.
Article18. Okumura M, Ohgushi H, Dohi Y, Katuda T, Tamai S, Koerten HK, et al. Osteoblastic phenotype expression on the surface of hydroxyapatite ceramics. J Biomed Mater Res. 1997. 37:122–129.
Article19. Deligianni DD, Katsala ND, Koutsoukos PG, Missirlis YF. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials. 2001. 22:87–96.
Article20. Wei G, Ma PX. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 2004. 25:4749–4757.
Article21. Jang YJ, Jung IH, Park JC, Jung UW, Kim CS, Lee YK, et al. Effect of seeding using an avidin-biotin binding system on the attachment of periodontal ligament fibroblasts to nanohydroxyapatite scaffolds: three-dimensional culture. J Periodontal Implant Sci. 2011. 41:73–78.
Article22. Park YS, Kim KN, Kim KM, Choi SH, Kim CK, Legeros RZ, et al. Feasibility of three-dimensional macroporous scaffold using calcium phosphate glass and polyurethane sponge. J Mater Sci. 2006. 41:4357–4364.
Article23. Kim CS, Choi SH, Chai JK, Cho KS, Moon IS, Wikesjo UM, et al. Periodontal repair in surgically created intrabony defects in dogs: influence of the number of bone walls on healing response. J Periodontol. 2004. 75:229–235.
Article24. Lee JS, Wikesjo UM, Jung UW, Choi SH, Pippig S, Siedler M, et al. Periodontal wound healing/regeneration following implantation of recombinant human growth/differentiation factor-5 in a beta-tricalcium phosphate carrier into one-wall intrabony defects in dogs. J Clin Periodontol. 2010. 37:382–389.
Article25. Cortellini P, Tonetti MS. Clinical performance of a regenerative strategy for intrabony defects: scientific evidence and clinical experience. J Periodontol. 2005. 76:341–350.26. Tonetti MS, Cortellini P, Lang NP, Suvan JE, Adriaens P, Dubravec D, et al. Clinical outcomes following treatment of human intrabony defects with GTR/bone replacement material or access flap alone. A multicenter randomized controlled clinical trial. J Clin Periodontol. 2004. 31:770–776.27. Urciuolo F, Imparato G, Guaccio A, Mele B, Netti PA. Novel strategies to engineering biological tissue in vitro. Methods Mol Biol. 2012. 811:223–244.
Article28. Kim JW, Choi KH, Yun JH, Jung UW, Kim CS, Choi SH, et al. Bone formation of block and particulated biphasic calcium phosphate lyophilized with Escherichia coli-derived recombinant human bone morphogenetic protein 2 in rat calvarial defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011. 112:298–306.
Article29. Schwarz F, Rothamel D, Herten M, Ferrari D, Sager M, Becker J. Lateral ridge augmentation using particulated or block bone substitutes biocoated with rhGDF-5 and rhBMP-2: an immunohistochemical study in dogs. Clin Oral Implants Res. 2008. 19:642–652.
Article30. Murakami S. Periodontal tissue regeneration by signaling molecule(s): what role does basic fibroblast growth factor (FGF-2) have in periodontal therapy? Periodontol 2000. 2011. 56:188–208.
Article31. Wikesjo UM, Sorensen RG, Kinoshita A, Jian Li X, Wozney JM. Periodontal repair in dogs: effect of recombinant human bone morphogenetic protein-12 (rhBMP-12) on regeneration of alveolar bone and periodontal attachment. J Clin Periodontol. 2004. 31:662–670.
Article32. Kawase T, Okuda K, Kogami H, Nakayama H, Nagata M, Sato T, et al. Human periosteum-derived cells combined with superporous hydroxyapatite blocks used as an osteogenic bone substitute for periodontal regenerative therapy: an animal implantation study using nude mice. J Periodontol. 2010. 81:420–427.
Article33. De Angelis N, Scivetti M. Lateral ridge augmentation using an equine flex bone block infused with recombinant human platelet-derived growth factor BB: a clinical and histologic study. Int J Periodontics Restorative Dent. 2011. 31:383–388.
Article34. Araujo M, Linder E, Lindhe J. Effect of a xenograft on early bone formation in extraction sockets: an experimental study in dog. Clin Oral Implants Res. 2009. 20:1–6.
Article35. Araujo MG, Liljenberg B, Lindhe J. Dynamics of Bio-Oss Collagen incorporation in fresh extraction wounds: an experimental study in the dog. Clin Oral Implants Res. 2010. 21:55–64.
Article36. Carmagnola D, Berglundh T, Araujo M, Albrektsson T, Lindhe J. Bone healing around implants placed in a jaw defect augmented with Bio-Oss. An experimental study in dogs. J Clin Periodontol. 2000. 27:799–805.
Article37. Carmagnola D, Berglundh T, Lindhe J. The effect of a fibrin glue on the integration of Bio-Oss with bone tissue. A experimental study in labrador dogs. J Clin Periodontol. 2002. 29:377–383.
Article38. Wikesjo UM, Claffey N, Egelberg J. Periodontal repair in dogs. Effect of heparin treatment of the root surface. J Clin Periodontol. 1991. 18:60–64.
Article39. Wikesjo UM, Lim WH, Thomson RC, Hardwick WR. Periodontal repair in dogs: gingival tissue occlusion, a critical requirement for GTR? J Clin Periodontol. 2003. 30:655–664.40. Araújo MG, Berglundh T, Lindhe J. On the dynamics of periodontal tissue formation in degree III furcation defects. An experimental study in dogs. J Clin Periodontol. 1997. 24:738–746.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Periodontal regenerative effect of a bovine hydroxyapatite/collagen block in one-wall intrabony defects in dogs: a histometric analysis
- Periodontal regeneration with nano-hyroxyapatite-coated silk scaffolds in dogs
- The Effects of calcium sulfate on healing of 1-wall intrabony defects in dogs
- The effects of Acellular dermal matrix on the healing of 1 wall intrabony defects in dogs
- The Effect of Calcium Sulfate on the Periodontal Healing of 2-Wall Intrabony Defects in Dogs