J Periodontal Implant Sci.
2011 Dec;41(6):285-292. 10.5051/jpis.2011.41.6.285.
Periodontal regenerative effect of a bovine hydroxyapatite/collagen block in one-wall intrabony defects in dogs: a histometric analysis
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. shchoi726@yuhs.ac
- KMID: 2094725
- DOI: http://doi.org/10.5051/jpis.2011.41.6.285
Abstract
- PURPOSE
The aim of this study was to elucidate the effect of a bovine hydroxyapatite/collagen (BHC) block in one-wall intrabony periodontal defects in dogs.
METHODS
A one-wall intrabony periodontal defect (4 mm wide and 5 mm deep) was prepared bilaterally at the mesial side of the mandibular fourth premolar in five beagle dogs. After thorough root planing, block-type BHC (4x5x5 mm) was placed on one side. The contralateral defect area did not receive any material as a sham-surgery control. Histological analysis of the sites was performed after an 8-week healing period.
RESULTS
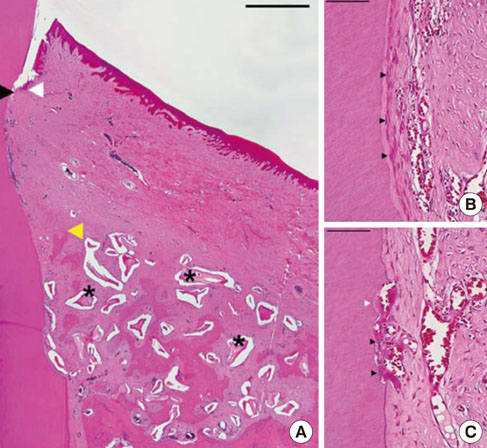
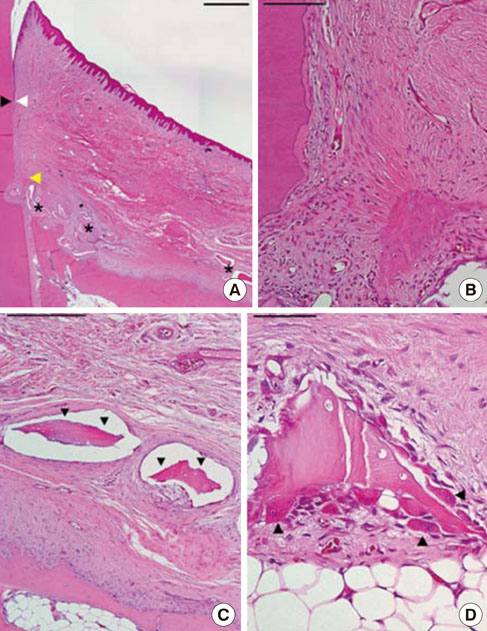
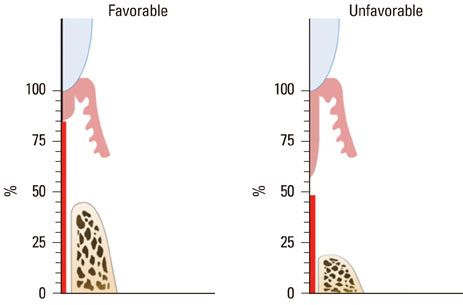
Two of five samples in the experimental group healed well without dissipation of the graft materials, and histological analysis revealed excellent regeneration of the periodontal tissues. However, most of the grafted materials had been displaced in the other three samples, leaving only a small portion of the graft. The measured parameters exhibited large standard deviations, and the mean values did not differ significantly between the experimental and sham-surgery control sides.
CONCLUSIONS
The application of BHC alone-without a barrier membrane-to wide, one-wall intrabony periodontal defects yielded inconsistent results regarding both periodontal regeneration and substantivity of the graft materials. Thus, the use of a barrier membrane for noncontained-type defects is recommended to improve the stability of the grafted material, and to condense it.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Role of collagen membrane in lateral onlay grafting with bovine hydroxyapatite incorporated with collagen matrix in dogs
Ui-Won Jung, Jung-Seok Lee, Geun Lee, In-Kyeong Lee, Ji-Wan Hwang, Min-Soo Kim, Seong-Ho Choi, Jung-Kiu Chai
J Periodontal Implant Sci. 2013;43(2):64-71. doi: 10.5051/jpis.2013.43.2.64.The effect of overlaying titanium mesh with collagen membrane for ridge preservation
Hyun-Chang Lim, Jung-Seok Lee, Seong-Ho Choi, Ui-Won Jung
J Periodontal Implant Sci. 2015;45(4):128-135. doi: 10.5051/jpis.2015.45.4.128.
Reference
-
1. Gottlow J, Nyman S, Karring T, Lindhe J. New attachment formation as the result of controlled tissue regeneration. J Clin Periodontol. 1984. 11:494–503.
Article2. Haney JM, Nilvéus RE, McMillan PJ, Wikesjö UM. Periodontal repair in dogs: expanded polytetrafluoroethylene barrier membranes support wound stabilization and enhance bone regeneration. J Periodontol. 1993. 64:883–890.
Article3. Sigurdsson TJ, Hardwick R, Bogle GC, Wikesjö UM. Periodontal repair in dogs: space provision by reinforced ePTFE membranes enhances bone and cementum regeneration in large supraalveolar defects. J Periodontol. 1994. 65:350–356.
Article4. Murphy KG. Postoperative healing complications associated with Gore-Tex Periodontal Material. Part I. Incidence and characterization. Int J Periodontics Restorative Dent. 1995. 15:363–375.5. Houser BE, Mellonig JT, Brunsvold MA, Cochran DL, Meffert RM, Alder ME. Clinical evaluation of anorganic bovine bone xenograft with a bioabsorbable collagen barrier in the treatment of molar furcation defects. Int J Periodontics Restorative Dent. 2001. 21:161–169.6. Paolantonio M. Combined periodontal regenerative technique in human intrabony defects by collagen membranes and anorganic bovine bone. A controlled clinical study. J Periodontol. 2002. 73:158–166.
Article7. Misch CE, Dietsh F. Bone-grafting materials in implant dentistry. Implant Dent. 1993. 2:158–167.
Article8. Taheri M, Molla R, Radvar M, Sohrabi K, Najafi MH. An evaluation of bovine derived xenograft with and without a bioabsorbable collagen membrane in the treatment of mandibular Class II furcation defects. Aust Dent J. 2009. 54:220–227.
Article9. Trombelli L, Heitz-Mayfield LJ, Needleman I, Moles D, Scabbia A. A systematic review of graft materials and biological agents for periodontal intraosseous defects. J Clin Periodontol. 2002. 29:Suppl 3. 117–135.
Article10. Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, Gunsolley JC. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann Periodontol. 2003. 8:227–265.
Article11. Blumenthal N, Steinberg J. The use of collagen membrane barriers in conjunction with combined demineralized bone-collagen gel implants in human infrabony defects. J Periodontol. 1990. 61:319–327.
Article12. Nygaard-Østby P, Bakke V, Nesdal O, Nilssen HK, Susin C, Wikesjö UM. Periodontal healing following reconstructive surgery: effect of guided tissue regeneration using a bioresorbable barrier device when combined with autogenous bone grafting. A randomized controlled clinical trial. J Clin Periodontol. 2008. 35:37–43.
Article13. Parashis A, Andronikaki-Faldami A, Tsiklakis K. Comparison of 2 regenerative procedures--guided tissue regeneration and demineralized freeze-dried bone allograft--in the treatment of intrabony defects: a clinical and radiographic study. J Periodontol. 1998. 69:751–758.
Article14. Berglundh T, Lindhe J. Healing around implants placed in bone defects treated with Bio-Oss. An experimental study in the dog. Clin Oral Implants Res. 1997. 8:117–124.
Article15. Camelo M, Nevins ML, Schenk RK, Simion M, Rasperini G, Lynch SE, et al. Clinical, radiographic, and histologic evaluation of human periodontal defects treated with Bio-Oss and Bio-Gide. Int J Periodontics Restorative Dent. 1998. 18:321–331.16. Richardson CR, Mellonig JT, Brunsvold MA, McDonnell HT, Cochran DL. Clinical evaluation of Bio-Oss: a bovine-derived xenograft for the treatment of periodontal osseous defects in humans. J Clin Periodontol. 1999. 26:421–428.
Article17. Mellonig JT. Human histologic evaluation of a bovine-derived bone xenograft in the treatment of periodontal osseous defects. Int J Periodontics Restorative Dent. 2000. 20:19–29.18. Yamada S, Shima N, Kitamura H, Sugito H. Effect of porous xenographic bone graft with collagen barrier membrane on periodontal regeneration. Int J Periodontics Restorative Dent. 2002. 22:389–397.19. Sculean A, Stavropoulos A, Windisch P, Keglevich T, Karring T, Gera I. Healing of human intrabony defects following regenerative periodontal therapy with a bovine-derived xenograft and guided tissue regeneration. Clin Oral Investig. 2004. 8:70–74.
Article20. Clergeau LP, Danan M, Clergeau-Guérithault S, Brion M. Healing response to anorganic bone implantation in periodontal intrabony defects in dogs. Part I. Bone regeneration. A microradiographic study. J Periodontol. 1996. 67:140–149.
Article21. Nevins ML, Camelo M, Lynch SE, Schenk RK, Nevins M. Evaluation of periodontal regeneration following grafting intrabony defects with bio-oss collagen: a human histologic report. Int J Periodontics Restorative Dent. 2003. 23:9–17.22. Zitzmann NU, Rateitschak-Plüss E, Marinello CP. Treatment of angular bone defects with a composite bone grafting material in combination with a collagen membrane. J Periodontol. 2003. 74:687–694.
Article23. Kim TG, Wikesjö UM, Cho KS, Chai JK, Pippig SD, Siedler M, et al. Periodontal wound healing/regeneration following implantation of recombinant human growth/differentiation factor-5 (rhGDF-5) in an absorbable collagen sponge carrier into one-wall intrabony defects in dogs: a dose-range study. J Clin Periodontol. 2009. 36:589–597.
Article24. Kim CS, Choi SH, Chai JK, Cho KS, Moon IS, Wikesjö UM, et al. Periodontal repair in surgically created intrabony defects in dogs: influence of the number of bone walls on healing response. J Periodontol. 2004. 75:229–235.
Article25. Kim CS, Um YJ, Chai JK, Cho KS, Moon IS, Choi SH, et al. A canine model for histometric evaluation of periodontal regeneration. Periodontol 2000. 2011. 56:209–226.
Article26. Stavropoulos A, Wikesjö UM. Influence of defect dimensions on periodontal wound healing/regeneration in intrabony defects following implantation of a bovine bone biomaterial and provisions for guided tissue regeneration: an experimental study in the dog. J Clin Periodontol. 2010. 37:534–543.
Article27. Araújo MG, Liljenberg B, Lindhe J. Dynamics of Bio-Oss Collagen incorporation in fresh extraction wounds: an experimental study in the dog. Clin Oral Implants Res. 2010. 21:55–64.
Article28. McAllister BS, Haghighat K. Bone augmentation techniques. J Periodontol. 2007. 78:377–396.
Article29. Esposito M, Piattelli M, Pistilli R, Pellegrino G, Felice P. Sinus lift with guided bone regeneration or anorganic bovine bone: 1-year post-loading results of a pilot randomised clinical trial. Eur J Oral Implantol. 2010. 3:297–305.30. Hämmerle CH, Chiantella GC, Karring T, Lang NP. The effect of a deproteinized bovine bone mineral on bone regeneration around titanium dental implants. Clin Oral Implants Res. 1998. 9:151–162.
Article31. Cardaropoli G, Araújo M, Hayacibara R, Sukekava F, Lindhe J. Healing of extraction sockets and surgically produced - augmented and non-augmented - defects in the alveolar ridge. An experimental study in the dog. J Clin Periodontol. 2005. 32:435–440.
Article32. Stavropoulos A, Kostopoulos L, Nyengaard JR, Karring T. Deproteinized bovine bone (Bio-Oss) and bioactive glass (Biogran) arrest bone formation when used as an adjunct to guided tissue regeneration (GTR): an experimental study in the rat. J Clin Periodontol. 2003. 30:636–643.
Article33. Araújo M, Linder E, Lindhe J. Effect of a xenograft on early bone formation in extraction sockets: an experimental study in dog. Clin Oral Implants Res. 2009. 20:1–6.
Article34. Sakata J, Abe H, Ohazama A, Okubo K, Nagashima C, Suzuki M, et al. Effects of combined treatment with porous bovine inorganic bone grafts and bilayer porcine collagen membrane on refractory one-wall intrabony defects. Int J Periodontics Restorative Dent. 2006. 26:161–169.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Calcium Sulfate on the Periodontal Healing of 2-Wall Intrabony Defects in Dogs
- The correlation of bone probing, radiographic and histometric measurements
- Periodontal tissue reaction to customized nano-hydroxyapatite block scaffold in one-wall intrabony defect: a histologic study in dogs
- Role of collagen membrane in lateral onlay grafting with bovine hydroxyapatite incorporated with collagen matrix in dogs
- Evaluating intra- and inter-examiner reproducibility in histometric measurement: one-wall intrabony periodontal defects in beagle dogs