Immune Netw.
2012 Feb;12(1):18-26. 10.4110/in.2012.12.1.18.
The Analysis of Vitamin C Concentration in Organs of Gulo-/- Mice Upon Vitamin C Withdrawal
- Affiliations
-
- 1Department of Anatomy, Medical Research Center, Seoul National University, Seoul 110-799, Korea. genius29@snu.ac.kr
- 2Institutes of Complementary and Integrative Medicine, Medical Research Center, Seoul National University, Seoul 110-799, Korea.
- KMID: 2014652
- DOI: http://doi.org/10.4110/in.2012.12.1.18
Abstract
- BACKGROUND
Vitamin C is an essential nutrient for maintaining human life. Vitamin C insufficiency in the plasma is closely related with the development of scurvy. However, in vivo kinetics of vitamin C regarding its storage and consumption is still largely unknown.
METHODS
We used Gulo-/- mice, which cannot synthesize vitamin C like human. Vitamin C level in plasma and organs from Gulo-/- mice was examined, and it compared with the level of wild-type mice during 5 weeks.
RESULTS
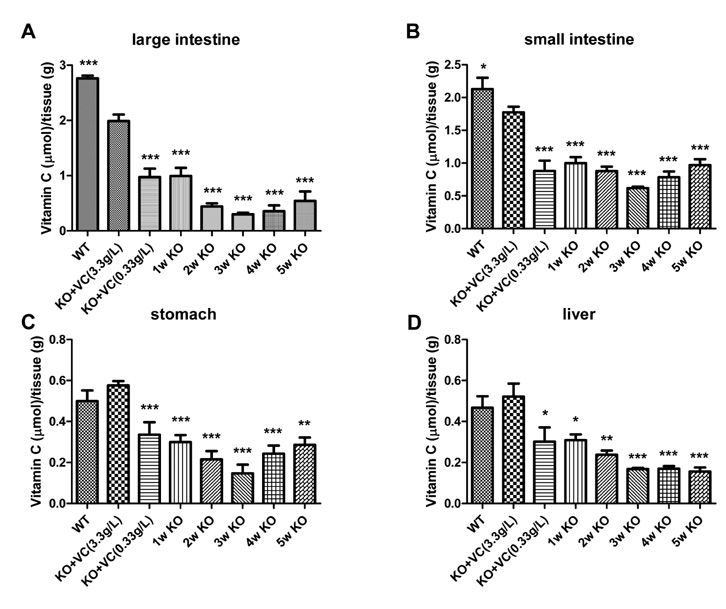
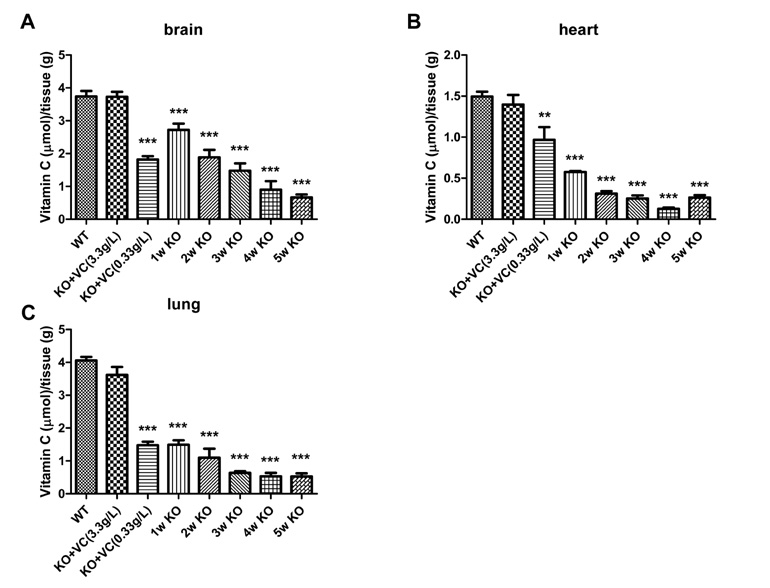
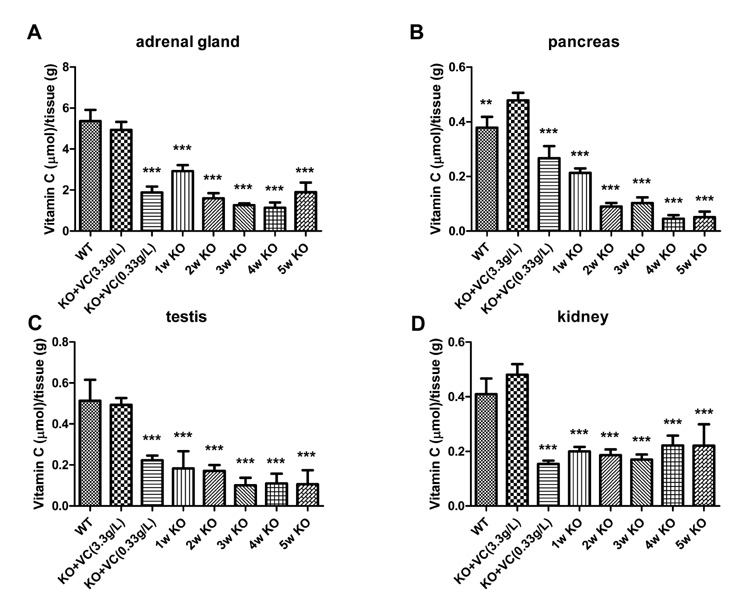
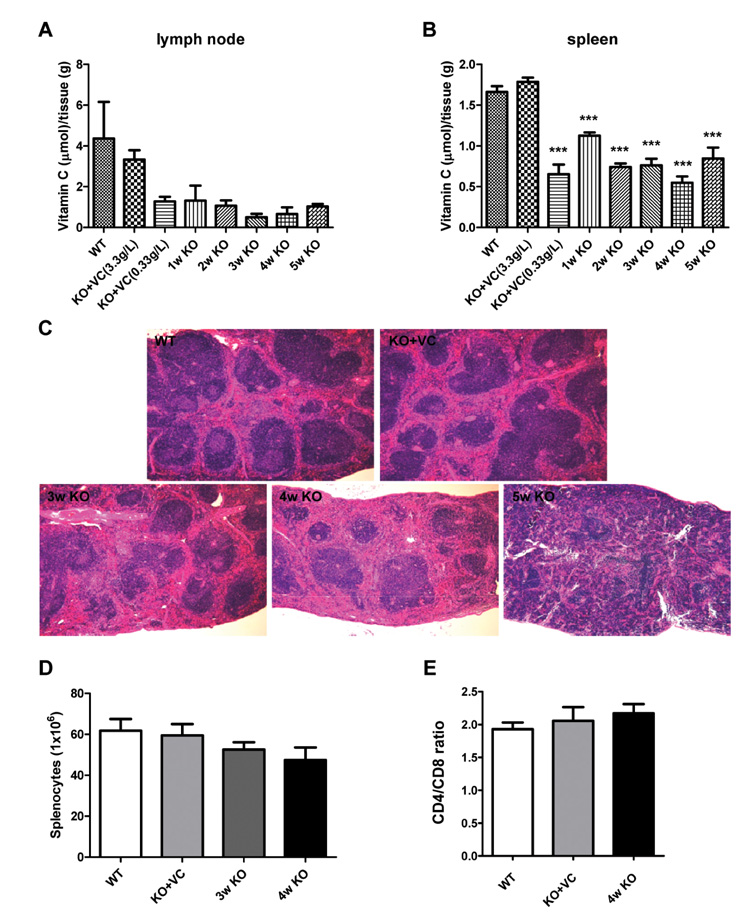
The significant weight loss of Gulo-/- mice was shown at 3 weeks after vitamin C withdrawal. However, there was no differences between wild-type and vitamin C-supplemented Gulo-/- mice (3.3 g/L in drinking water). The concentration of vitamin C in plasma and organs was significantly decreased at 1 week after vitamin C withdrawal. Vitamin C is preferentially deposited in adrenal gland, lymph node, lung, and brain. There were no significant changes in the numbers and CD4/CD8 ratio of splenocytes in Gulo-/- mice with vitamin C withdrawal for 4 weeks. And the architecture of spleen in Gulo-/- mice was disrupted at 5 weeks after vitamin C withdrawal.
CONCLUSION
The vitamin C level of Gulo-/- mice was considerably decreased from 1 week after vitamin C withdrawal. Vitamin C is preferentially stored in some organs such as brain, adrenal gland and lung.
MeSH Terms
Figure
Cited by 2 articles
-
Vitamin C Up-regulates Expression of CD80, CD86 and MHC Class II on Dendritic Cell Line, DC-1 Via the Activation of p38 MAPK
Hyung Woo Kim, Su In Cho, Seyeon Bae, Hyemin Kim, Yejin Kim, Young-il Hwang, Jae Seung Kang, Wang Jae Lee
Immune Netw. 2012;12(6):277-283. doi: 10.4110/in.2012.12.6.277.Vitamin C Is an Essential Factor on the Anti-viral Immune Responses through the Production of Interferon-α/β at the Initial Stage of Influenza A Virus (H3N2) Infection
Yejin Kim, Hyemin Kim, Seyeon Bae, Jiwon Choi, Sun Young Lim, Naeun Lee, Joo Myung Kong, Young-il Hwang, Jae Seung Kang, Wang Jae Lee
Immune Netw. 2013;13(2):70-74. doi: 10.4110/in.2013.13.2.70.
Reference
-
1. Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999. 69:1086–1107.
Article2. Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003. 22:18–35.
Article3. Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989. 86:6377–6381.
Article4. Ellis GR, Anderson RA, Lang D, Blackman DJ, Morris RH, Morris-Thurgood J, McDowell IF, Jackson SK, Lewis MJ, Frenneaux MP. Neutrophil superoxide anion--generating capacity, endothelial function and oxidative stress in chronic heart failure: effects of short- and long-term vitamin C therapy. J Am Coll Cardiol. 2000. 36:1474–1482.
Article5. Sauberlich HE. Packer L, Fuchs J, editors. A history of scurvy and vitamin C. Vitamin C in health and disease. 1997. New York: Marcel Dekker;1–24.6. Wintergerst ES, Maggini S, Hornig DH. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006. 50:85–94.
Article7. Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A. 1976. 73:3685–3689.
Article8. Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, Khosh DB, Drisko J, Levine M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci U S A. 2008. 105:11105–11109.
Article9. Nishikimi M, Kawai T, Yagi K. Guinea pigs possess a highly mutated gene for L-gulono-gamma-lactone oxidase, the key enzyme for L-ascorbic acid biosynthesis missing in this species. J Biol Chem. 1992. 267:21967–21972.
Article10. Nishikimi M, Fukuyama R, Minoshima S, Shimizu N, Yagi K. Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. J Biol Chem. 1994. 269:13685–13688.
Article11. Burns JJ. Missing step in man, monkey and guinea pig required for the biosynthesis of L-ascorbic acid. Nature. 1957. 180:553.
Article12. Maeda N, Hagihara H, Nakata Y, Hiller S, Wilder J, Reddick R. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci U S A. 2000. 97:841–846.
Article13. Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A. 1996. 93:3704–3709.
Article14. Harrison FE, Green RJ, Dawes SM, May JM. Vitamin C distribution and retention in the mouse brain. Brain Res. 2010. 1348:181–186.
Article15. Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999. 399:70–75.
Article16. Linster CL, Van Schaftingen E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2007. 274:1–22.17. Englard S, Seifter S. The biochemical functions of ascorbic acid. Annu Rev Nutr. 1986. 6:365–406.
Article18. Koike K, Kondo Y, Sekiya M, Sato Y, Tobino K, Iwakami SI, Goto S, Takahashi K, Maruyama N, Seyama K, Ishigami A. Complete lack of vitamin C intake generates pulmonary emphysema in senescence marker protein-30 knockout mice. Am J Physiol Lung Cell Mol Physiol. 2010. 298:L784–L792.
Article19. Parsons KK, Maeda N, Yamauchi M, Banes AJ, Koller BH. Ascorbic acid-independent synthesis of collagen in mice. Am J Physiol Endocrinol Metab. 2006. 290:E1131–E1139.
Article20. Chojkier M, Spanheimer R, Peterkofsky B. Specifically decreased collagen biosynthesis in scurvy dissociated from an effect on proline hydroxylation and correlated with body weight loss. In vitro studies in guinea pig calvarial bones. J Clin Invest. 1983. 72:826–835.
Article21. Peterkofsky B. Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy. Am J Clin Nutr. 1991. 54:6 Suppl. 1135S–1140S.
Article22. Lee SK, Kang JS, Jung da J, Hur DY, Kim JE, Hahm E, Bae S, Kim HW, Kim D, Cho BJ, Cho D, Shin DH, Hwang YI, Lee WJ. Vitamin C suppresses proliferation of the human melanoma cell SK-MEL-2 through the inhibition of cyclooxygenase-2 (COX-2) expression and the modulation of insulin-like growth factor II (IGF-II) production. J Cell Physiol. 2008. 216:180–188.
Article23. Odumosu A. Anorectic drugs and vitamin C: role in appetite and brain ascorbic acid in guineapigs. Int J Vitam Nutr Res. 1981. 51:247–253.24. Kang JS, Cho D, Kim YI, Hahm E, Kim YS, Jin SN, Kim HN, Kim D, Hur D, Park H, Hwang YI, Lee WJ. Sodium ascorbate (vitamin C) induces apoptosis in melanoma cells via the down-regulation of transferrin receptor dependent iron uptake. J Cell Physiol. 2005. 204:192–197.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vitamin C Is an Essential Factor on the Anti-viral Immune Responses through the Production of Interferon-alpha/beta at the Initial Stage of Influenza A Virus (H3N2) Infection
- Differentially expressed proteins in the liver of Gulo-/- mice following treatments with Helicobacter pylori and diethylnitrosamine
- Affinity for 57Co-Vitamin B12 by a Wide Histologic Variety of Tumor Types in Mice
- Ascorbic acid insufficiency induces the severe defect on bone formation via the down-regulation of osteocalcin production
- Vitamin D: A View of Perinatal Medicine