Immune Netw.
2012 Feb;12(1):8-17. 10.4110/in.2012.12.1.8.
Baculovirus-based Vaccine Displaying Respiratory Syncytial Virus Glycoprotein Induces Protective Immunity against RSV Infection without Vaccine-Enhanced Disease
- Affiliations
-
- 1Division of Life & Pharmaceutical Sciences, and Center for Cell Signaling & Drug Discovery Research, Ewha Womans University, Seoul 120-750, Korea. tcell@ewha.ac.kr
- KMID: 2014651
- DOI: http://doi.org/10.4110/in.2012.12.1.8
Abstract
- BACKGROUND
Respiratory syncytial virus (RSV) is a major cause of severe lower respiratory tract diseases in infancy and early childhood. Despite its importance as a pathogen, there is no licensed vaccine against RSV yet. The attachment glycoprotein (G) of RSV is a potentially important target for protective antiviral immune responses. Recombinant baculovirus has been recently emerged as a new vaccine vector, since it has intrinsic immunostimulatory properties and good bio-safety profile.
METHODS
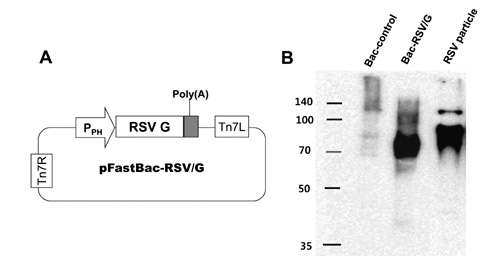
We have constructed a recombinant baculovirus-based RSV vaccine, Bac-RSV/G, displaying G glycoprotein, and evaluated immunogenicity and protective efficacy by intranasal immunization of BALB/c mice with Bac-RSV/G.
RESULTS
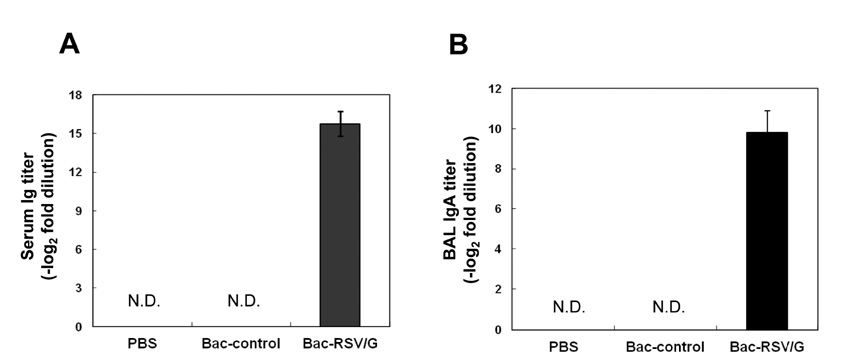
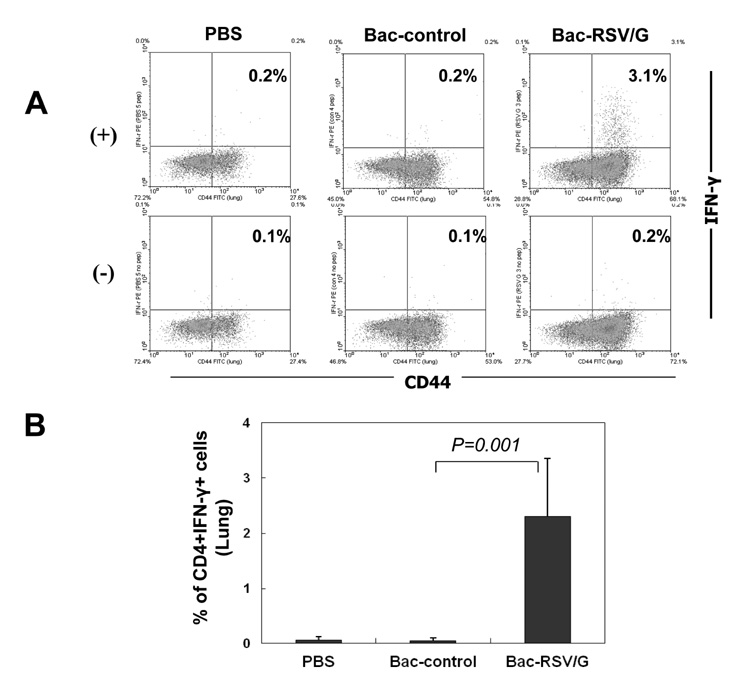
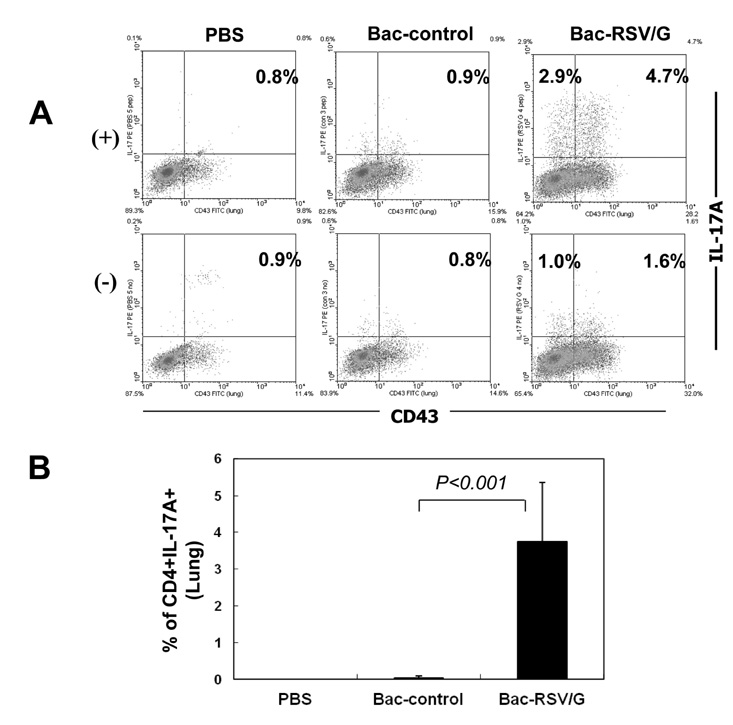
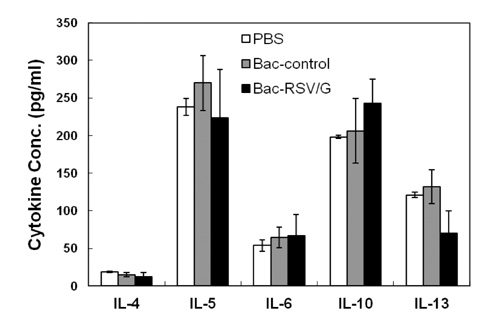
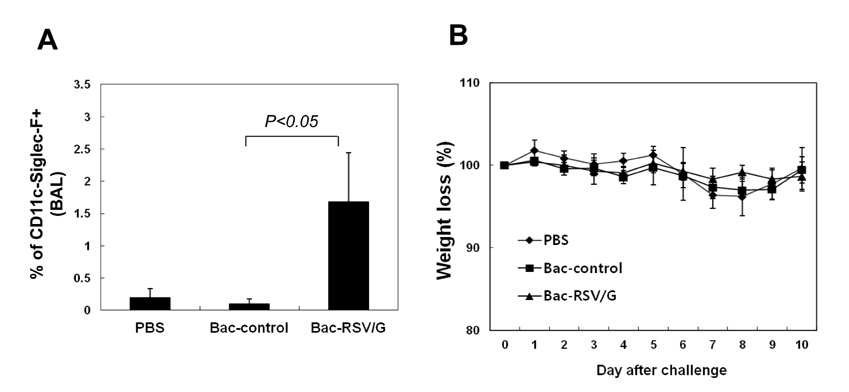
Bac-RSV/G efficiently provides protective immunity against RSV challenge. Strong serum IgG and mucosal IgA responses were induced by intranasal immunization with Bac-RSV/G. In addition to humoral immunity, G-specific Th17- as well as Th1-type T-cell responses were detected in the lungs of Bac-RSV/G-immune mice upon RSV challenge. Neither lung eosinophilia nor vaccine-induced weight loss was observed upon Bac-RSV/G immunization and subsequent RSV infection.
CONCLUSION
Our data demonstrate that intranasal administration of baculovirus-based Bac-RSV/G vaccine is efficient for the induction of protection against RSV and represents a promising prophylactic vaccination regimen.
MeSH Terms
Figure
Cited by 2 articles
-
Immunogenicity and Protective Efficacy of a Dual Subunit Vaccine Against Respiratory Syncytial Virus and Influenza Virus
Min-Hee Park, Jun Chang
Immune Netw. 2012;12(6):261-268. doi: 10.4110/in.2012.12.6.261.Vaccine containing G protein fragment and recombinant baculovirus expressing M2 protein induces protective immunity to respiratory syncytial virus
Yeong-Min Jo, Jungwoo Kim, Jun Chang
Clin Exp Vaccine Res. 2019;8(1):43-53. doi: 10.7774/cevr.2019.8.1.43.
Reference
-
1. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005. 352:1749–1759.
Article2. Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000. 13:371–384.
Article3. Robinson RF. Impact of respiratory syncytial virus in the United States. Am J Health Syst Pharm. 2008. 65:23 Suppl 8. S3–S6.
Article4. Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000. 283:499–505.
Article5. Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969. 89:435–448.6. Openshaw PJ, Clarke SL, Record FM. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int Immunol. 1992. 4:493–500.
Article7. Varga SM, Wang X, Welsh RM, Braciale TJ. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4(+) T cells. Immunity. 2001. 15:637–646.
Article8. Tebbey PW, Hagen M, Hancock GE. Atypical pulmonary eosinophilia is mediated by a specific amino acid sequence of the attachment (G) protein of respiratory syncytial virus. J Exp Med. 1998. 188:1967–1972.
Article9. Yu JR, Kim S, Lee JB, Chang J. Single intranasal immunization with recombinant adenovirus-based vaccine induces protective immunity against respiratory syncytial virus infection. J Virol. 2008. 82:2350–2357.
Article10. Blissard GW, Rohrmann GF. Baculovirus diversity and molecular biology. Annu Rev Entomol. 1990. 35:127–155.
Article11. Kost TA, Condreay JP, Jarvis DL. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol. 2005. 23:567–575.
Article12. O'Reilly DR, Miller LK, Luckow VA. Baculovirus expression vectors: a laboratory manual. 1992. Oxford (UK): Oxford University Press.13. Lee MJ, Jin YH, Kim K, Choi Y, Kim HC, Park S. Expression of hepatitis B virus x protein in hepatocytes suppresses CD8 T cell activity. Immune Netw. 2010. 10:126–134.
Article14. Bai B, Lu X, Meng J, Hu Q, Mao P, Lu B, Chen Z, Yuan Z, Wang H. Vaccination of mice with recombinant baculovirus expressing spike or nucleocapsid protein of SARS-like coronavirus generates humoral and cellular immune responses. Mol Immunol. 2008. 45:868–875.
Article15. Strauss R, Hüser A, Ni S, Tuve S, Kiviat N, Sow PS, Hofmann C, Lieber A. Baculovirus-based vaccination vectors allow for efficient induction of immune responses against plasmodium falciparum circumsporozoite protein. Mol Ther. 2007. 15:193–202.
Article16. Abe T, Takahashi H, Hamazaki H, Miyano-Kurosaki N, Matsuura Y, Takaku H. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J Immunol. 2003. 171:1133–1139.
Article17. Barsoum J, Brown R, McKee M, Boyce FM. Efficient transduction of mammalian cells by a recombinant baculovirus having the vesicular stomatitis virus G glycoprotein. Hum Gene Ther. 1997. 8:2011–2018.
Article18. Pieroni L, Maione D, La Monica N. In vivo gene transfer in mouse skeletal muscle mediated by baculovirus vectors. Hum Gene Ther. 2001. 12:871–881.19. Facciabene A, Aurisicchio L, La Monica N. Baculovirus vectors elicit antigen-specific immune responses in mice. J Virol. 2004. 78:8663–8672.
Article20. Park SH, Chang J, Yang SH, Kim HJ, Kwak HH, Kim BM, Lee SH. Enhancement of antigen-specific antibody and CD8(+) T cell responses by codelivery of IL-12-encapsulated microspheres in protein and peptide vaccination. Immune Netw. 2007. 7:186–196.
Article21. Lee JB, Chang J. CD43 Expression regulated by IL-12 signaling is associated with survival of CD8 T cells. Immune Netw. 2010. 10:153–163.
Article22. Brandtzaeg P. Role of secretory antibodies in the defence against infections. Int J Med Microbiol. 2003. 293:3–15.
Article23. Hancock GE, Speelman DJ, Heers K, Bortell E, Smith J, Cosco C. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of respiratory syncytial virus. J Virol. 1996. 70:7783–7791.
Article24. Srikiatkhachorn A, Braciale TJ. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997. 186:421–432.
Article25. Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J Immunol Methods. 2007. 327:63–74.
Article26. Chang J. Current progress on development of respiratory syncytial virus vaccine. BMB Rep. 2011. 44:232–237.
Article27. Alwan WH, Kozlowska WJ, Openshaw PJ. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994. 179:81–89.
Article28. Lin YH, Lee LH, Shih WL, Hu YC, Liu HJ. Baculovirus surface display of sigmaC and sigmaB proteins of avian reovirus and immunogenicity of the displayed proteins in a mouse model. Vaccine. 2008. 26:6361–6367.
Article29. Yoshida S, Kondoh D, Arai E, Matsuoka H, Seki C, Tanaka T, Okada M, Ishii A. Baculovirus virions displaying Plasmodium berghei circumsporozoite protein protect mice against malaria sporozoite infection. Virology. 2003. 316:161–170.
Article30. Yang DG, Chung YC, Lai YK, Lai CW, Liu HJ, Hu YC. Avian influenza virus hemagglutinin display on baculovirus envelope: cytoplasmic domain affects virus properties and vaccine potential. Mol Ther. 2007. 15:989–996.
Article31. Hu YC. Baculovirus as a highly efficient expression vector in insect and mammalian cells. Acta Pharmacol Sin. 2005. 26:405–416.
Article32. Gronowski AM, Hilbert DM, Sheehan KC, Garotta G, Schreiber RD. Baculovirus stimulates antiviral effects in mammalian cells. J Virol. 1999. 73:9944–9951.
Article33. Abe T, Kaname Y, Wen X, Tani H, Moriishi K, Uematsu S, Takeuchi O, Ishii KJ, Kawai T, Akira S, Matsuura Y. Baculovirus induces type I interferon production through toll-like receptor-dependent and -independent pathways in a cell-type-specific manner. J Virol. 2009. 83:7629–7640.
Article34. Tripp RA, Oshansky C, Alvarez R. Cytokines and respiratory syncytial virus infection. Proc Am Thorac Soc. 2005. 2:147–149.
Article35. Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004. 21:467–476.
Article36. Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009. 27:485–517.
Article37. Yeh N, Glosson NL, Wang N, Guindon L, McKinley C, Hamada H, Li Q, Dutton RW, Shrikant P, Zhou B, Brutkiewicz RR, Blum JS, Kaplan MH. Tc17 cells are capable of mediating immunity to vaccinia virus by acquisition of a cytotoxic phenotype. J Immunol. 2010. 185:2089–2098.
Article38. McKinstry KK, Strutt TM, Buck A, Curtis JD, Dibble JP, Huston G, Tighe M, Hamada H, Sell S, Dutton RW, Swain SL. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009. 182:7353–7363.
Article39. Newcomb DC, Zhou W, Moore ML, Goleniewska K, Hershey GK, Kolls JK, Peebles RS Jr. A functional IL-13 receptor is expressed on polarized murine CD4+ Th17 cells and IL-13 signaling attenuates Th17 cytokine production. J Immunol. 2009. 182:5317–5321.
Article40. Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006. 203:2715–2725.
Article41. Jaffar Z, Ferrini ME, Herritt LA, Roberts K. Cutting edge: lung mucosal Th17-mediated responses induce polymeric Ig receptor expression by the airway epithelium and elevate secretory IgA levels. J Immunol. 2009. 182:4507–4511.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In Hot Pursuit of the First Vaccine Against Respiratory Syncytial Virus
- Vaccine containing G protein fragment and recombinant baculovirus expressing M2 protein induces protective immunity to respiratory syncytial virus
- Recent Advances in the Prevention of RSV in Neonates and Young Infants
- Immunogenicity and Protective Efficacy of a Dual Subunit Vaccine Against Respiratory Syncytial Virus and Influenza Virus
- Respiratory Syncytial Virus Infection Complicated by Extrapulmonary Manifestations