Immune Netw.
2012 Dec;12(6):261-268. 10.4110/in.2012.12.6.261.
Immunogenicity and Protective Efficacy of a Dual Subunit Vaccine Against Respiratory Syncytial Virus and Influenza Virus
- Affiliations
-
- 1Division of Life & Pharmaceutical Sciences, Ewha Womans University, Seoul 120-750, Korea. tcell@ewha.ac.kr
- KMID: 2150758
- DOI: http://doi.org/10.4110/in.2012.12.6.261
Abstract
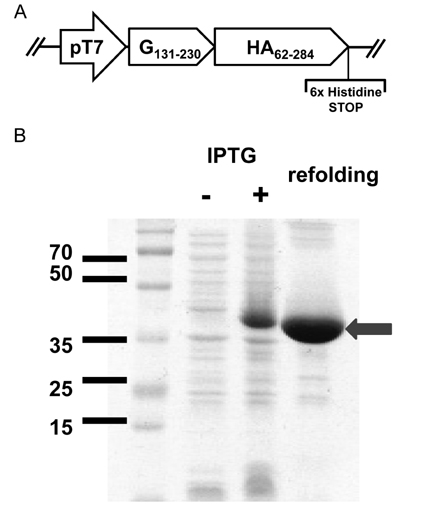
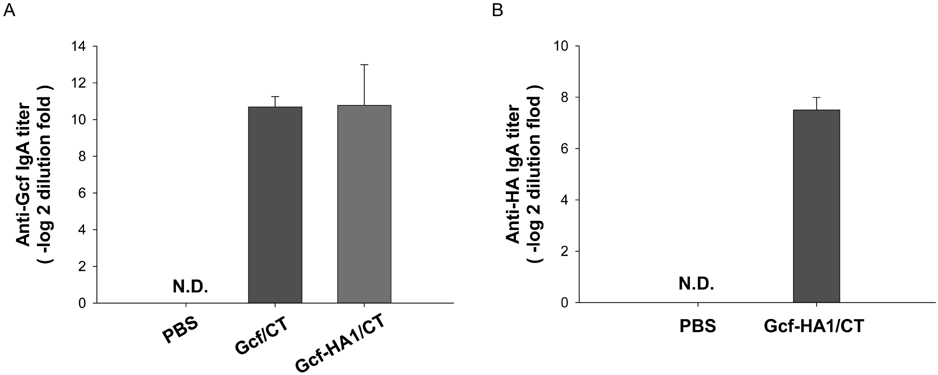
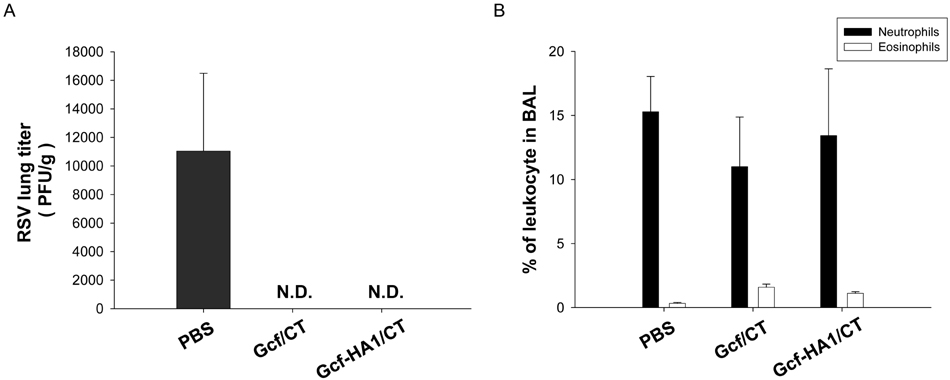
- Respiratory syncytial virus (RSV) and influenza virus are the most significant pathogens causing respiratory tract diseases. Composite vaccines are useful in reducing the number of vaccination and confer protection against multiple infectious agents. In this study, we generated fusion of RSV G protein core fragment (amino acid residues 131 to 230) and influenza HA1 globular head domain (amino acid residues 62 to 284) as a dual vaccine candidate. This fusion protein, Gcf-HA1, was bacterially expressed, purified by metal resin affinity chromatography, and refolded in PBS. BALB/c mice were intranasally immunized with Gcf-HA1 in combination with a mucosal adjuvant, cholera toxin (CT). Both serum IgG and mucosal IgA responses specific to Gcf and HA1 were significantly increased in Gcf-HA1/CT-vaccinated mice. To determine the protective efficacy of Gcf-HA1/CT vaccine, immunized mice were challenged with RSV (A2 strain) or influenza virus (A/PR/8/34). Neither detectable viral replication nor pathology was observed in the lungs of the immune mice. These results demonstrate that immunity induced by intranasal Gcf-HA1/CT immunization confers complete protection against both RSV and homologous influenza virus infection, suggesting our Gcf-HA1 vaccine candidate could be further developed as a dual subunit vaccine against RSV and influenza virus.
Keyword
MeSH Terms
-
Animals
Cholera Toxin
Chorionic Gonadotropin, beta Subunit, Human
Chromatography, Affinity
GTP-Binding Proteins
Head
Hemagglutinins
Immunization
Immunoglobulin A
Immunoglobulin G
Influenza, Human
Lung
Mice
Orthomyxoviridae
Peptide Fragments
Respiratory Syncytial Viruses
Respiratory Tract Diseases
Vaccination
Vaccines
Cholera Toxin
Chorionic Gonadotropin, beta Subunit, Human
GTP-Binding Proteins
Hemagglutinins
Immunoglobulin A
Immunoglobulin G
Peptide Fragments
Vaccines
Figure
Reference
-
1. Falsey AR, Walsh EE. Respiratory syncytial virus infection in elderly adults. Drugs Aging. 2005. 22:577–587.
Article2. Murphy BR, Walsh EE. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J Clin Microbiol. 1988. 26:1595–1597.
Article3. Connors M, Giese NA, Kulkarni AB, Firestone CY, Morse HC 3rd, Murphy BR. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994. 68:5321–5325.
Article4. Kim HW, Leikin SL, Arrobio J, Brandt CD, Chanock RM, Parrott RH. Cell-mediated immunity to respiratory syncytial virus induced by inactivated vaccine or by infection. Pediatr Res. 1976. 10:75–78.
Article5. Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol. 1996. 70:2852–2860.
Article6. Collins PL, Purcell RH, London WT, Lawrence LA, Chanock RM, Murphy BR. Evaluation in chimpanzees of vaccinia virus recombinants that express the surface glycoproteins of human respiratory syncytial virus. Vaccine. 1990. 8:164–168.
Article7. Jang JE, Lee JB, Kim KH, Park SM, Shim BS, Cheon IS, Song MK, Chang J. Evaluation of protective efficacy of respiratory syncytial virus vaccine against A and B subgroup human isolates in Korea. PLoS ONE. 2011. 6:e23797.
Article8. Yu JR, Kim S, Lee JB, Chang J. Single intranasal immunization with recombinant adenovirus-based vaccine induces protective immunity against respiratory syncytial virus infection. J Virol. 2008. 82:2350–2357.
Article9. Dormitzer PR, Galli G, Castellino F, Golding H, Khurana S, Del Giudice G, Rappuoli R. Influenza vaccine immunology. Immunol Rev. 2011. 239:167–177.
Article10. Kim S, Joo DH, Lee JB, Shim BS, Cheon IS, Jang JE, Song HH, Kim KH, Song MK, Chang J. Dual role of respiratory syncytial virus glycoprotein fragment as a mucosal immunogen and chemotactic adjuvant. PLoS One. 2012. 7:e32226.
Article11. Lee JB, Jang JE, Song MK, Chang J. Intranasal delivery of cholera toxin induces th17-dominated T-cell response to bystander antigens. PLoS One. 2009. 4:e5190.
Article12. Kim S, Chang J. Baculovirus-based vaccine displaying respiratory syncytial virus glycoprotein induces protective immunity against RSV infection without vaccine-enhanced disease. Immune Netw. 2012. 12:8–17.
Article13. Bembridge GP, Rodriguez N, Garcia-Beato R, Nicolson C, Melero JA, Taylor G. Respiratory syncytial virus infection of gene gun vaccinated mice induces Th2-driven pulmonary eosinophilia even in the absence of sensitisation to the fusion (F) or attachment (G) protein. Vaccine. 2000. 19:1038–1046.
Article14. Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol. 2001. 2:732–738.
Article15. Plotnicky-Gilquin H, Goetsch L, Huss T, Champion T, Beck A, Haeuw JF, Nguyen TN, Bonnefoy JY, Corvaïa N, Power UF. Identification of multiple protective epitopes (protectopes) in the central conserved domain of a prototype human respiratory syncytial virus G protein. J Virol. 1999. 73:5637–5645.
Article16. Caton AJ, Brownlee GG, Yewdell JW, Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell. 1982. 31:417–427.
Article17. Khurana S, Larkin C, Verma S, Joshi MB, Fontana J, Steven AC, King LR, Manischewitz J, McCormick W, Gupta RK, Golding H. Recombinant HA1 produced in E. coli forms functional oligomers and generates strain-specific SRID potency antibodies for pandemic influenza vaccines. Vaccine. 2011. 29:5657–5665.
Article18. Song L, Nakaar V, Kavita U, Price A, Huleatt J, Tang J, Jacobs A, Liu G, Huang Y, Desai P, Maksymiuk G, Takahashi V, Umlauf S, Reiserova L, Bell R, Li H, Zhang Y, McDonald WF, Powell TJ, Tussey L. Efficacious recombinant influenza vaccines produced by high yield bacterial expression: a solution to global pandemic and seasonal needs. PLoS One. 2008. 3:e2257.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recovery of respiratory syncytial virus, adenovirus, influenza virus , and parainfluenza virus from nasopharyngeal aspirates from children with acute respiratory tract infections
- In Hot Pursuit of the First Vaccine Against Respiratory Syncytial Virus
- Need for a safe vaccine against respiratory syncytial virus infection
- Simultaneous Detection and Identification of Human Respiratory Syncytial Virus, Influenza Virus A ( H3N2 , H1N1 ) and B by One - tube Multiplex Reverse Transcription Polymerase Chain Reaction
- A Case of Severe Influenza Infection in a Child with Nephrotic Syndrome on Steroid Therapy