J Korean Diabetes Assoc.
2007 Jan;31(1):1-8. 10.4093/jkda.2007.31.1.1.
The Roles of Clusterin on Morphogenesis of Beta Cells During Pancreas Regeneration
- Affiliations
-
- 1Department of Anatomy and Center for Advanced Medical Education by BK21 project1, College of Medicine, Inha University, Korea.
- 2Department of Pharmacology & BK21 program for Medical Sciences2, College of Medicine, Korea University, Korea.
- KMID: 2008091
- DOI: http://doi.org/10.4093/jkda.2007.31.1.1
Abstract
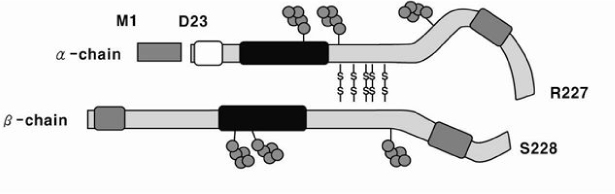
- Clusterin is a highly glycosylated heterodimeric glycoprotein that plays diverse biological roles in various organs. The secreted clusterin has been established as a major form of the protein that exerts diverse tissue effects. For instance, clusterin is known to act in cell protection through the actions of extra-cellular molecular chaperones. In the extracellular milieu, clusterin participates in specific interactions with a diverse array of native biological molecules including LRP-2 (Lipoprotein receptor-related protein 2, also known as gp330 or megalin), which is involved in ligand endocytosis at the surfaces of certain epithelia. Clusterin is expressed transiently in developing and differentiating endocrine pancreatic cells and might be involved in pancreas development. This transient expression of clusterin at specific time points of pancreas development and cell differentiation during pancreas regeneration implies that the protein is a regulatory factor for cytodifferentiation as well as for replication. A specific action of the clusterin in the reconstruction and remodeling of the endocrine pancreas has been demonstrated. It also strongly stimulates duct cell differentiation into insulin-secreting cells under in vitro culture conditions. Clusterin appears thus as a potent regulator of insulin cell morphogenesis.
Keyword
MeSH Terms
Figure
Reference
-
1. Hsieh SY, Chen WY, Shih TC, Yeh JY, Jeng JT. Dys-regulation of clusterin in human hepatoma is not associated with tumorigenesis but is secondary to cell response to external tresses. Mol Carcinog. 2005. 43:100–107.2. O'Sullivan J, Whyte L, Drake J, Tenniswood M. Alterations in the post-translational modification and intracellular trafficking of clusterin in MCF-7 cells during apoptosis. Cell Death Differ. 2003. 10:914–927.3. Blaschuk O, Burdzy K, Fritz IB. Purification and characterization of a cell-aggregating factor (clusterin), the major glycoprotein in ram rete testis fluid. J Biol Chem. 1983. 258:7714–7720.4. Fritz IB, Burdzy K, Setchell B, Balschuk O. Ram rete testis fluid contains a protein (Clusterin) which influences cell-cell interactions in vitro. Biology of Reproduction. 1983. 28:1283–1388.5. Slawin K, Sawczuk IS, Olsson CA, Buttyan R. Chromosomal assignment of the human homologue encoding SGP-2. Biochem Biophys Res Commun. 1990. 172(1):160–164.6. Collard MW, Griswold MD. Biosynthesis and molecular cloning of sulfated glycoprotein 2 secreted by rat Sertoli cells. Biochemistry. 1987. 26:3297–3303.7. Leger JG, Montpetit ML, Tenniswood MP. Characterization and cloning of androgen-repressed mRNAs from rat ventral prostate. Biochem Biophys Res Commun. 1987. 147:196–203.8. May PC, Johnson SA, Poirier J, Lampert-Etchells M, Finch CE. Altered gene expression in Alzheimer's disease brain tissue. Can J Neurol Sci. 1989. 16:473–476.9. Duguid JR, Bohmont CW, Liu N, Tourtellotte WW. Changes in brain gene expression shared by scrapie and Alzheimer disease. Proc Natl Acad Sci U S A. 1989. 86:7260–7264.10. DeSilva HV, Stuart WD, Duvic CR, Wetterau JR, Ray MJ, Ferguson DG, Albers HW, Smith WR, Harmony JAK. A 70-kDa apolipoprotein designated ApoJ is a marker for subclasses of human plasma high density lipoproteins. J Biol Chem. 1990. 265:13240–13247.11. Yang CR, Leskov K, Hosley-Eberlein K, Criswell T, Pink JJ, Kinsella TJ, Boothman DA. Nuclear clusterin/XIP8, an x-ray-induced Ku70-binding protein that signals cell death. Proc Natl Acad Sci U S A. 2000. 97:5907–5912.12. Purrello M, Bettuzzi S, Di Pietro C, Mirabile E, Di Blasi M, Rimini R, Grzeschik KH, Ingletti C, Corti A, Sichel G. The gene for SP-40,40, human homolog of rat sulfated glycoprotein 2, rat clusterin, and rat testosterone-repressed prostate message 2, maps to chromosome 8. Genomics. 1991. 10:151–156.13. Tobe T, Minoshima S, Yamase S, Choi NH, Tomita M, Shimizu N. Assignment of a human serum glycoprotein SP-40,40 gene (CLI) to chromosome 8. Cytogenet Cell Genet. 1991. 57:193–195.14. Wong P, Taillefer D, Lakins J, Pineault J, Chader G, Tenniswood M. Molecular characterization of human TRPM-2/clusterin, a gene associated with sperm maturation, apoptosis and neurodegeneration. Eur J Biochem. 1994. 221:917–925.15. Junod A, Lambert AE, Orci L, Pictet R, Gonet AE, Renold AE. Studies of the diabetogenic action of streptozotocin. Proc Soc Exp Biol Med. 1967. 26:201–205.16. Cantenys D, Portha B, Dutrillaux MC, Hollande E, Roze C, Picon L. Histogenesis of the endocrine pancreas in newborn rats after destruction by streptozotocin. An immunocytochemical study. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981. 35:109–122.17. Like AA, Appel MC, Williams RM, Rossini AA. Streptozotocin-induced pancreatic insulitis in mice. Morphologic and physiologic studies. Lab Invest. 1978. 38:470–486.18. Bonnevie-Nielsen V, Steffes MW, Lernmark A. A major loss in islet mass and B-cell function precedes hyperglycemia in mice given multiple low doses of streptozotocin. Diabetes. 1981. 30:424–429.19. Park IS, Bendayan M. Characterization of the endocrine cells in the pancreatic-bile duct system of the rat. Anat Rec. 1992. 232:247–256.20. Bouwens L, Braet F, Heimberg H. Identification of rat pancreatic duct cells by their expression of cytokeratins 7, 19, and 20 in vivo and after isolation and culture. J Histochem Cytochem. 1995. 43:245–253.21. Hellerström C, Swenne I, Anderson A. Lefebvre PJ, Pipeleers DG, editors. Islet cell replication and diabetes. The Pathology of the Endocrine Pancreas in Diabetes. 1998. Heidelberg, Germany: Springer-Verlag;141–170.22. Plachot C, Movassat J, Portha B. Impaired beta-cell regeneration after partial pancreatectomy in the adult Goto-Kakizaki rat, a spontaneous model of type II diabetes. Histochem Cell Biol. 2001. 116:131–139.23. Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999. 48:2270–2276.24. Rafaeloff R, Pittenger GL, Barlow SW, Qin XF, Yan B, Rosenberg L, Duguid WP, Vinik AI. Cloning and sequencing of the pancreatic islet neogenesis associated protein (INGAP) gene and its expression in islet neogenesis in hamsters. J Clin Invest. 1997. 99:2100–2109.25. Min BH, Kim BM, Lee SH, Kang SW, Bendayan M, Park IS. Clusterin expression in the early process of pancreas regeneration in the pancreatectomized rat. J Histochem Cytochem. 2003. 51:1355–1365.26. Calvo EL, Bernatchez G, Pelletier G, Iovanna JL, Morisset J. Downregulation of IGF-I mRNA expression during postnatal pancreatic development and overexpression after subtotal pancreatectomy and acute pancreatitis in the rat pancreas. J Mol Endocrinol. 1997. 18:233–242.27. Scaglia L, Smith FE, Bonner-Weir S. Apoptosis contributes to the involution of beta cell mass in the post partum rat pancreas. Endocrinology. 1995. 136:5461–5468.28. Kim BM, Kim SY, Lee S, Shin YJ, Min BH, Bendayan M, Park IS. Clusterin induces differentiation of pancreatic duct cells into insulin-secreting cells. Diabetologia. 2006. 49:311–320.29. Jin G, Howe PH. Regulation of clusterin gene expression by transforming growth factor beta. J Biol Chem. 1997. 272:26620–26626.30. Suh E, Wang Z, Swain GP, Tenniswood M, Traber PG. Clusterin gene transcription is activated by caudal-related homeobox genes in intestinal epithelium. Am J Physiol Gastrointest Liver Physiol. 2001. 280:G149–G156.31. Schedin P, Mitrenga T, Kaeck M. Estrous cycle regulation of mammary epithelial cell proliferation, differentiation, and death in the Sprague-Dawley rat: a model for investigating the role of estrous cycling in mammary carcinogenesis. J Mammary Gland Biol Neoplasia. 2000. 5:211–225.32. French LE, Chonn A, Ducrest D, et al. Murine clusterin: molecular cloning and mRNA localization of a gene associated with epithelial differentiation processes during embryogenesis. J Cell Biol. 1993. 122:1119–1123.33. Jin G, Howe PH. Transforming growth factor beta regulates clusterin gene expression via modulation of transcription factor c-Fos. Eur J Biochem. 1999. 263:534–542.34. Gutacker C, Klock G, Diel P, Koch-Brandt C. Nerve growth factor and epidermal growth factor stimulate clusterin gene expression in PC12 cells. Biochem J. 1999. 339:759–766.35. Reddy KB, Jin G, Karode MC, Harmony JA, Howe PH. Transforming growth factor beta (TGF beta)-induced nuclear localization of apolipoproteinJ/clusterin in epithelial cells. Biochemistry. 1996. 35:6157–6163.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Pancreatic Tissue Regeneration and Molecular Regulators
- Pancreatic Exocrine and Endocrine Cell Differentiation during Pancreatic Regeneration
- Stimualtion of Beta Cell Neogenesis by SBTI (Soybean Trypsin Inhibitor) or Soybean Diet in the Subtotal Pancreatectomy Models
- Stem Cell In Adult Pancreas: Its Activation and Induction of Beta Cell Differentiation
- Proapoptotic role of nuclear clusterin in brain