Anat Cell Biol.
2012 Jun;45(2):114-120. 10.5115/acb.2012.45.2.114.
Arginine vasopressin (AVP) expressional changes in the hypothalamic paraventricular and supraoptic nuclei of stroke-prone spontaneously hypertensive rats
- Affiliations
-
- 1Department of Biomedical Laboratory Science, College of Biomedical Sciences, Soonchunhyang University, Asan, Korea.
- 2Life Science Research Institute, Unigen Inc., Cheonan, Korea.
- 3Department of Laboratory Animal Research, Central Research Institute, Dr. Chung's Food Co. Ltd., Cheongju, Korea.
- 4Department of Anatomy and Cell Biology, College of Veterinary Medicine and Research Institute for Veterinary Science, Seoul National University, Seoul, Korea. ysyoon@snu.ac.kr
- KMID: 2005874
- DOI: http://doi.org/10.5115/acb.2012.45.2.114
Abstract
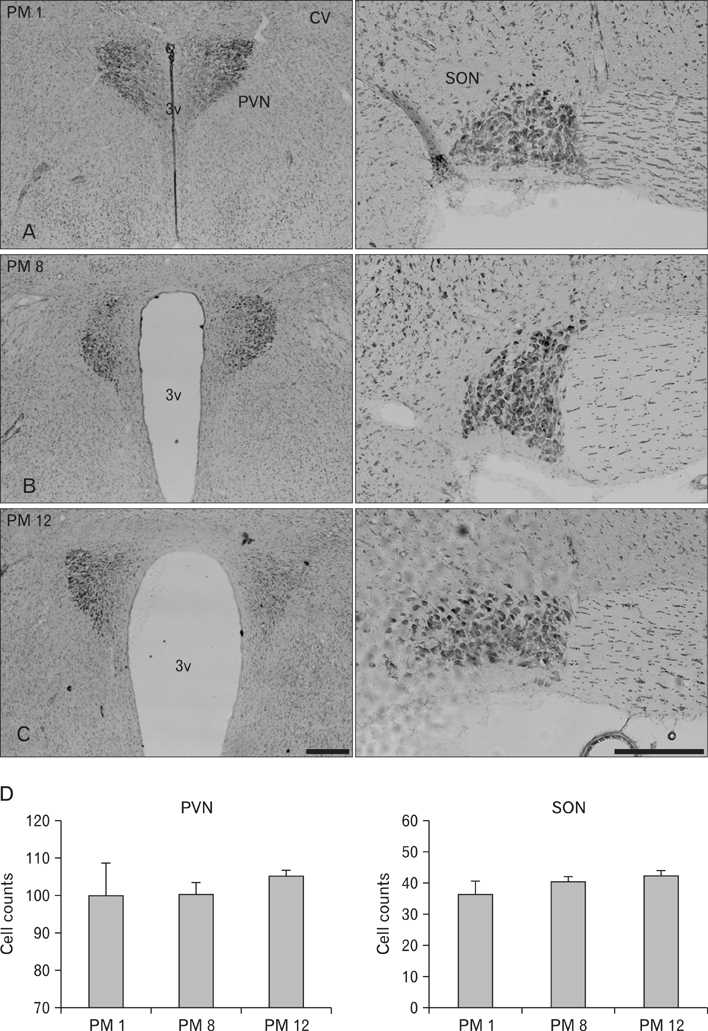
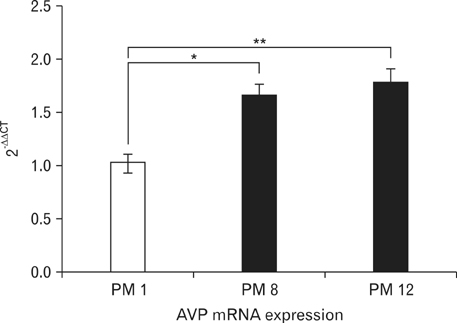
- Arginine vasopressin (AVP) is a neuropeptide with vasoconstrictive, antidiuretic, cardiovascular regulative and hepatic glycogenolysis effects, that also affects other behaviors including modulating learning. A number of studies on AVP regulation have been conducted in various metabolic diseases (disorders). In this study, the immunoreactivities of AVP in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) and mRNA expressions in the hypothalamus were investigated by immunohistochemistry and quantitative real-time PCR (RT-qPCR) in stroke-prone spontaneously hypertensive rats at different ages (i.e., at postnatal months [PM] 1, 8, and 12). Blood glucose levels in the PM 8 group were higher than in the other groups. However, cresyl violet positive neurons were detected in the PVN and SON of all animals, and numbers of cresyl violet positive neurons were similar in all aged groups. In addition, AVP immunoreactivity was detected in the PVN and SON of all age groups, and AVP immunoreactivity and mRNA expression levels were found to be increased in proportion to age by immunohistochemistry and RT-qPCR. These results suggest that the diabetic condition is temporally generated after hypertension has developed. Furthermore, our findings suggest that increased AVP expressions in the hypothalamic PVN and SON are associated with hypertension by age.
Keyword
MeSH Terms
-
Aged
Animals
Arginine
Arginine Vasopressin
Benzoxazines
Blood Glucose
Glycogenolysis
Humans
Hypertension
Hypothalamus
Immunohistochemistry
Learning
Metabolic Diseases
Molybdenum
Neurons
Neuropeptides
Oxides
Paraventricular Hypothalamic Nucleus
Rats, Inbred SHR
Real-Time Polymerase Chain Reaction
RNA, Messenger
Supraoptic Nucleus
Viola
Arginine
Arginine Vasopressin
Benzoxazines
Blood Glucose
Molybdenum
Neuropeptides
Oxides
RNA, Messenger
Figure
Reference
-
1. Jansen K, Van der Zee EA, Gerkema MP. Vasopressin immunoreactivity, but not vasoactive intestinal polypeptide, correlates with expression of circadian rhythmicity in the suprachiasmatic nucleus of voles. Neuropeptides. 2007. 41:207–216.2. Keppens S, de Wulf H. The activation of liver glycogen phosphorylase by vasopressin. FEBS Lett. 1975. 51:29–32.3. Mlynarik M, Zelena D, Bagdy G, Makara GB, Jezova D. Signs of attenuated depression-like behavior in vasopressin deficient Brattleboro rats. Horm Behav. 2007. 51:395–405.4. Gaida W, Lang RE, Kraft K, Unger T, Ganten D. Altered neuropeptide concentrations in spontaneously hypertensive rats: cause or consequence? Clin Sci (Lond). 1985. 68:35–43.5. Jablonskis LT, Rogers PF, Lungershausen YK, Howe PR. Chronic central administration of enalaprilat lowers blood pressure in stroke-prone spontaneously hypertensive rats. J Auton Nerv Syst. 1992. 39:119–126.6. Jablonskis LT, Howe PR. Vasopressin compensates for acute loss of sympathetic pressor tone in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 1993. 20:380–383.7. Rogers PF, Head GA, Lungershausen YK, Howe PR. Effects of depleting central and peripheral adrenaline stores on blood pressure in stroke-prone spontaneously hypertensive rats. J Auton Nerv Syst. 1991. 34:9–16.8. Song J, Martin DS. Rho kinase contributes to androgen amplification of renal vasoconstrictor responses in the spon taneously hypertensive rat. J Cardiovasc Pharmacol. 2006. 48:103–109.9. Kodama N, Komuta K, Nanba H. Can maitake MD-fraction aid cancer patients? Altern Med Rev. 2002. 7:236–239.10. Morita M, Kita Y, Morikawa N, Iwami M, Notsu Y. Expression of arginine vasopressin and vasopressin V1a receptor mRNA in diabetic (db/db) mice. Exp Clin Endocrinol Diabetes. 2001. 109:261–266.11. Shimamoto K, Ura N. Mechanisms of insulin resistance in hypertensive rats. Clin Exp Hypertens. 2006. 28:543–552.12. Zelena D, Filaretova L, Mergl Z, Barna I, Tóth ZE, Makara GB. Hypothalamic paraventricular nucleus, but not vasopressin, participates in chronic hyperactivity of the HPA axis in diabetic rats. Am J Physiol Endocrinol Metab. 2006. 290:E243–E250.13. Zelena D, Mergl Z, Makara GB. The role of vasopressin in diabetes mellitus-induced hypothalamo-pituitary-adrenal axis activation: studies in Brattleboro rats. Brain Res Bull. 2006. 69:48–56.14. Pak TR, Chung WC, Hinds LR, Handa RJ. Arginine vasopressin regulation in pre- and postpubertal male rats by the androgen metabolite 3beta-diol. Am J Physiol Endocrinol Metab. 2009. 296:E1409–E1413.15. Zhu H, Huang Q, Xu H, Niu L, Zhou JN. Antidepressant-like effects of sodium butyrate in combination with estrogen in rat forced swimming test: involvement of 5-HT(1A) receptors. Behav Brain Res. 2009. 196:200–206.16. Nabika T, Cui Z, Masuda J. The stroke-prone spontaneously hypertensive rat: how good is it as a model for cerebrovascular diseases? Cell Mol Neurobiol. 2004. 24:639–646.17. Kase CS, Mohr JP, Caplan LR. Barnett HJ, Mohr JP, Stein BM, Yatsu FM, editors. Intracerebral hemorrhage. Stroke: Pathophysiology, Diagnosis, and Management. 1998. New York: Churchill Livingstone;649–700.18. Mohr JP, Marti-Vilalta JL. Barnett HJ, Mohr JP, Stein BM, Yatsu FM, editors. 1998. Lacunes. Stroke: Pathophysiology, Diagnosis, and Management. 1998. New York: Churchill Livingstone;599–622.19. Fukuda S, Tsuchikura S, Iida H. Age-related changes in blood pressure, hematological values, concentrations of serum biochemical constituents and weights of organs in the SHR/Izm, SHRSP/Izm and WKY/Izm. Exp Anim. 2004. 53:67–72.20. Gomibuchi H, Okazaki M, Iwai S, Kumai T, Kobayashi S, Oguchi K. Development of hyperfibrinogenemia in spontaneously hypertensive and hyperlipidemic rats: a potentially useful animal model as a complication of hypertension and hyperlipidemia. Exp Anim. 2007. 56:1–10.21. Tomassoni D, Avola R, Di Tullio MA, Sabbatini M, Vitaioli L, Amenta F. Increased expression of glial fibrillary acidic protein in the brain of spontaneously hypertensive rats. Clin Exp Hypertens. 2004. 26:335–350.22. Yibchok-anun S, Abu-Basha EA, Yao CY, Panichkriangkrai W, Hsu WH. The role of arginine vasopressin in diabetes-associated increase in glucagon secretion. Regul Pept. 2004. 122:157–162.23. Makara GB, Mergl Z, Zelena D. The role of vasopressin in hypothalamo-pituitary-adrenal axis activation during stress: an assessment of the evidence. Ann N Y Acad Sci. 2004. 1018:151–161.24. Yi SS, Hwang IK, Shin JH, Choi JH, Lee CH, Kim IY, Kim YN, Won MH, Park IS, Seong JK, Yoon YS. Regulatory mechanism of hypothalamo-pituitary-adrenal (HPA) axis and neuronal changes after adrenalectomy in type 2 diabetes. J Chem Neuroanat. 2010. 40:130–139.25. Yi SS, Hwang IK, Kim YN, Kim IY, Pak SI, Lee IS, Seong JK, Yoon YS. Enhanced expressions of arginine vasopressin (Avp) in the hypothalamic paraventricular and supraoptic nuclei of type 2 diabetic rats. Neurochem Res. 2008. 33:833–841.26. Küchler S, Perwitz N, Schick RR, Klein J, Westphal S. Arginine-vasopressin directly promotes a thermogenic and pro-inflammatory adipokine expression profile in brown adipocytes. Regul Pept. 2010. 164:126–132.27. Luo Y, Kaur C, Ling EA. Neuronal and glial response in the rat hypothalamus-neurohypophysis complex with streptozotocin-induced diabetes. Brain Res. 2002. 925:42–54.28. Saravia FE, Gonzalez SL, Roig P, Alves V, Homo-Delarche F, De Nicola AF. Diabetes increases the expression of hypothalamic neuro peptides in a spontaneous model of type I diabetes, the nonobese diabetic (NOD) mouse. Cell Mol Neurobiol. 2001. 21:15–27.29. Pandey NR, Benkirane K, Amiri F, Schiffrin EL. Effects of PPAR-gamma knock-down and hyperglycemia on insulin signaling in vascular smooth muscle cells from hypertensive rats. J Cardiovasc Pharmacol. 2007. 49:346–354.30. Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007. 50:202–211.31. Ishida H, Takemori K, Dote K, Ito H. Expression of glucose transporter-1 and aquaporin-4 in the cerebral cortex of stroke-prone spontaneously hypertensive rats in relation to the blood-brain barrier function. Am J Hypertens. 2006. 19:33–39.32. Ueno M, Sakamoto H, Liao YJ, Onodera M, Huang CL, Miyanaka H, Nakagawa T. Blood-brain barrier disruption in the hypothalamus of young adult spontaneously hypertensive rats. Histochem Cell Biol. 2004. 122:131–137.33. Ueno M, Sakamoto H, Tomimoto H, Akiguchi I, Onodera M, Huang CL, Kanenishi K. Blood-brain barrier is impaired in the hippocampus of young adult spontaneously hypertensive rats. Acta Neuropathol. 2004. 107:532–538.34. O'Donnell ME, Duong V, Suvatne J, Foroutan S, Johnson DM. Arginine vasopressin stimulation of cerebral microvascular endothelial cell Na-K-Cl cotransporter activity is V1 receptor and [Ca] dependent. Am J Physiol Cell Physiol. 2005. 289:C283–C292.35. Kim J, Jung Y. Different expressions of AQP1, AQP4, eNOS, and VEGF proteins in ischemic versus non-ischemic cerebropathy in rats: potential roles of AQP1 and eNOS in hydrocephalic and vasogenic edema formation. Anat Cell Biol. 2011. 44:295–303.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Chronic Alcohol Intake on Vasopressin and Oxytocin-containing Neurons in the Paraventricular and Supraoptic Nucleus of the Rat Hypothalamus
- Changes of neuropeptides immunoreactivity according to dehydration in paraventricular nuclei
- A Study on the Appearance of Oxytocin- and Vasopressin-specific Neurons in the Human Fetus by Monoclonal Antibodies
- Changes In The Distribution of Oxytocin and Vasopressin-Immunoreactive Neurons In the Hypothalamic Area of Normal and Hypophysectomized Rats
- Effects of Dehydration on Vasopressin and Oxytocin Immunoreactive Neurons of the Hypothalamus in the Mongolian Gerbil