Anat Cell Biol.
2012 Jun;45(2):97-102. 10.5115/acb.2012.45.2.97.
Differential expression levels of synaptophysin through developmental stages in hippocampal region of mouse brain
- Affiliations
-
- 1Department of NanoBio Medical Science, Dankook University College of Medicine, Cheonan, Korea.
- 2Department of Anatomy, Dankook University College of Medicine, Cheonan, Korea. Anat104@dku.edu
- KMID: 2005872
- DOI: http://doi.org/10.5115/acb.2012.45.2.97
Abstract
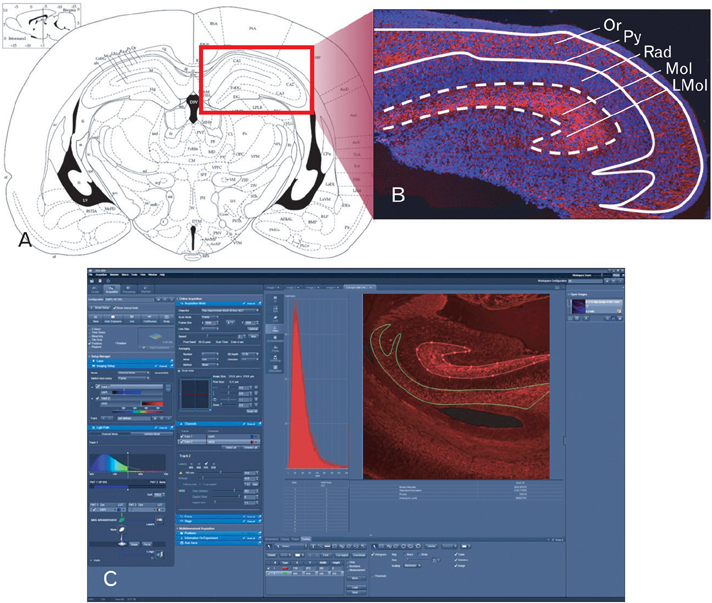
- The formation of neural synapses according to the development and growth of neurite were usually studied with various markers. Of these markers, synaptophysin is a kind of synaptic protein located in the synaptic vesicle of neuron or neuroendocrine cell known to be distributed consistently in all neural synapses. The purpose of this study was to investigate differential expression levels and patterns of synaptic marker (synaptophysin) in the mouse hippocampal region according to the developmental stages of embryonic, neonatal, and adulthood respectively. In the embryonic and neonatal groups, synaptophysin immunofluorescence was almost defined to cornu ammonis subfields (CA1 and CA3) of hippocampus and subiculum proper in the hippocampal region. However in dentate gyrus, synaptophysin immunoreactivities were insignificant or absent in all developmental stages. In embryonic and neonatal hippocampus, the intensities of immunofluorescence were significantly different between molecular and oriens layers. Furthermore, those intensities were decreased considerably in both layers of neonatal group compared to embryonic. The results from this study will contribute to characterizing synaptogenic activities in the central nervous system through developmental stages.
MeSH Terms
Figure
Reference
-
1. Eastwood SL, Harrison PJ. Synaptic pathology in the anterior cingulate cortex in schizophrenia and mood disorders. A review and a Western blot study of synaptophysin, GAP-43 and the complexins. Brain Res Bull. 2001. 55:569–578.2. Jin Y. Synaptogenesis. 2005. Pasadena: WormBook;1–11.3. Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985. 41:1017–1028.4. Sudhof TC, Lottspeich F, Greengard P, Mehl E, Jahn R. A synaptic vesicle protein with a novel cytoplasmic domain and four transmembrane regions. Science. 1987. 238:1142–1144.5. Leube RE. The topogenic fate of the polytopic transmembrane proteins, synaptophysin and connexin, is determined by their membrane-spanning domains. J Cell Sci. 1995. 108(Pt 3):883–894.6. Arthur CP, Stowell MH. Structure of synaptophysin: a hexameric MARVEL-domain channel protein. Structure. 2007. 15:707–714.7. Kwon SE, Chapman ER. Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron. 2011. 70:847–854.8. Pyeon HJ, Lee IL. Differential expression levels of synaptophysin through developmental stages in cerebral cortices of mouse brain. Korean J Phys Anthropol. 2012. 25:55–62.9. Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007. 11:379–386.10. VanElzakker M, Fevurly RD, Breindel T, Spencer RL. Environmental novelty is associated with a selective increase in Fos expression in the output elements of the hippocampal formation and the perirhinal cortex. Learn Mem. 2008. 15:899–908.11. Di Gennaro G, Grammaldo LG, Quarato PP, Esposito V, Mascia A, Sparano A, Meldolesi GN, Picardi A. Severe amnesia following bilateral medial temporal lobe damage occurring on two distinct occasions. Neurol Sci. 2006. 27:129–133.12. Rolls ET, Xiang JZ. Spatial view cells in the primate hippocampus and memory recall. Rev Neurosci. 2006. 17:175–200.13. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 1982. Sydney: Academic Press.14. Hsia AY, Malenka RC, Nicoll RA. Development of excitatory circuitry in the hippocampus. J Neurophysiol. 1998. 79:2013–2024.15. Groc L, Gustafsson B, Hanse E. Early establishment of multiple release site connectivity between interneurons and pyramidal neurons in the developing hippocampus. Eur J Neurosci. 2003. 17:1873–1880.16. Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002. 3:728–739.17. Riebe I, Hanse E. Development of synaptic connectivity onto interneurons in stratum radiatum in the CA1 region of the rat hippocampus. BMC Neurosci. 2012. 13:14.18. Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998. 4:1313–1317.19. Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990. 301:365–381.20. Choi JM, Romeo RD, Brake WG, Bethea CL, Rosenwaks Z, McEwen BS. Estradiol increases pre- and post-synaptic proteins in the CA1 region of the hippocampus in female rhesus macaques (Macaca mulatta). Endocrinology. 2003. 144:4734–4738.21. Bian C, Zhu K, Guo Q, Xiong Y, Cai W, Zhang J. Sex differences and synchronous development of steroid receptor coactivator-1 and synaptic proteins in the hippocampus of postnatal female and male C57BL/6 mice. Steroids. 2012. 77:149–156.22. Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, Muller SA, Rammner B, Grater F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmuller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006. 127:831–846.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Expression Levels of Synaptophysin through Developmental Stages in Cerebral Cortices of Mouse Brain
- Differential Expressions of Synaptogenic Markers between Primary Cultured Cortical and Hippocampal Neurons
- Effect of Developmental Lead Exposure on the Expression of Hippocampal NMDA Receptor Subunit mRNA
- Temporal changes in mammalian target of rapamycin (mTOR) and phosphorylated-mTOR expressions in the hippocampal CA1 region of rat with vascular dementia
- m6A Methyltransferase METTL3 Reduces Hippocampal Neuron Apoptosis in a Mouse Model of Autism Through the MALAT1/SFRP2/Wnt/β-catenin Axis