J Korean Acad Conserv Dent.
2004 Sep;29(5):479-484. 10.5395/JKACD.2004.29.5.479.
The effect of Treponema denticola immunoinhibitory protein on cytokine expression in T cells
- Affiliations
-
- 1Department of Conservative Dentistry, School of Dentistry, Seoul National University, Korea. limss@snu.ac.kr
- KMID: 1987128
- DOI: http://doi.org/10.5395/JKACD.2004.29.5.479
Abstract
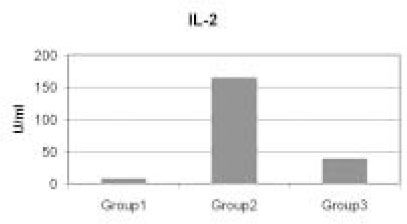
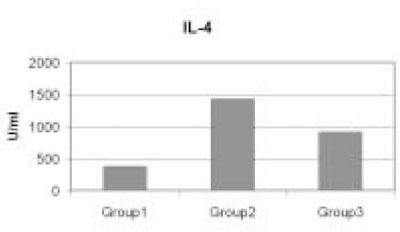
- Immunoinhibitory protein extracted from sonicated Treponema denticola have been shown to suppress cell cycle progression of human lymphocytes. To study in detail about the effect of this microorganism on the function of lymphocytes, we investigated the levels of Interleukin-2 (IL-2) and Interleukin-4 (IL-4) production by T lymphocytes before and after the addition of 12.5 microg/ml T. denticola sonicated extracts. In this study, levels of IL-2 and IL-4 produced from T cells pretreated with sonicated extracts were evaluated by using the quantitative sandwich enzyme immunoassay technique. In response to phytohemagglutinin (PHA) stimulation, T cell produced increased levels of IL-2 and IL-4. However, the expressions of both cytokines were significantly inhibited when PHA activated-T cells were pre-exposed to sonicated T. denticola extracts (p < 0.05). These findings suggest that the T. denticola sonicated extracts induced-immunosuppression in Th1 and Th2 cell functions could be a part of the pathogenic mechanism of the endodontic failure associated with this microorganism.
MeSH Terms
Figure
Reference
-
1. Loesche WJ. The role of spirochetes in periodontal disease. Adv Dent Res. 1988. 2:275–283.
Article2. Listgarten MA. Colonization of subgingival areas by motile rods and spirochetes: clinical implication in host-parasite interaction in periodontal diseases. 1982. Washington D.C.: American Society of Microbiology;112.3. Thilo B, Baehni P, Holz J, Baume LJ. Distribution des bacteries dans les parties coronaire et apicale de dents a pulpe necrosee. SSO Schweiz Monatsschr Zahnheilkd. 1983. 93:335–350.4. Ramachandran Nair PN. Light and electron microscopic studies of root canal flora and periapical lesions. J Endod. 1987. 13:29–39.
Article5. Siqueira JF Jr, Rocas IN, Favieri A, Santos KRN. Detection of Treponema denticola in endodontics infections by 16S rRNA gene detected polymerase chain reaction. Oral Microbiol Immunol. 2000. 15:335–337.
Article6. Siqueira JF Jr, Rocas IN, Oliveira JCM, Santos KRN. Detection of putative oral pathogens in acute periradicular abscesses by 16S rDNA-directed polymerase chain reaction. J Endod. 2001. 27:164–167.
Article7. Shenker BJ, Listgarten MA, Taichman NS. A monocyte-dependent phenomenon. J Immunol. 1984. 132:2039–2045.8. Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996. 383:787–793.
Article9. Stashenko P. The role of immune cytokines in the pathogenesis of periapical lesions. Endod Dent Traumatol. 1990. 6:89–96.
Article10. Harris JI, Russell RRB, Curtis MA, Aduse-Opoku J, Taylor JJ. Molecular mediators of Porphyromonas gingivalis-induced T-cell apoptosis. Oral Microbiol Immunol. 2002. 17:224–230.11. Shenker BJ, Datar S. Fusobacterium nucleatum inhibits human T-cells by arresting cells in the mid G1 phase of the cell cycle. Infect Immun. 1995. 63:4830–4836.
Article12. Lee W, Lim S, Son H, Bae K. Sonicated Extract of Enterococcus faecalis induces irreversible cell cycle arrest in phytohemagglutinin-activated human lymphocytes. J Endod. 2004. 30:209–212.
Article13. Yoshida H, Jontell M, Sundqvist G, Bergenholtz G. Effect of sonicated material from Fusobacterium nucleatum on the functional capacity of accessory cells derived from dental pulp. Oral Microbiol Immunol. 1995. 10:208–212.
Article14. Lee W, Pankoski L, Zekavat A, Shenker BJ. Treponema denticola imunoinhibitory protein induces irreversible G1 arrest in activated human lymphocytes. Oral Microbiol Immunol. 2004. 19:144–149.
Article15. Siqueira JF Jr, Rocas IN. Treponema socranskii in primary endodontic infections as detected by nested PCR. J Endod. 2003. 29:244–247.
Article16. Baumgartner JC, Khemaleelakul S, Xia T. Identification of spirochetes (Treponemes) in endodontic infections. J Endod. 2003. 29:794–797.
Article17. Jung IY, Choi B, Kum K, Roh B, Lee S, Lee C, Park D. Molecular epidermiology and association of putative pathogens in root canal infection. J Endod. 2000. 26:599–604.
Article18. Smallwood E, Gharbia SE, Gulabivala K, Shah HN. Isolation and direct nucleic acid detection of oral spirochetes in root canal infections. Int Endo J. 1998. 31:189–220.19. Chan EC, McLaughlin R. Taxonomy and virulence of oral spirochetes. Oral Microbiol Immunol. 2000. 15:1–9.
Article20. Kesavalu L, Walker S, Holt S, Crawley R, Eversole J. Virulence characteristics of oral treponemes in a murine model. Infect Immun. 1997. 65:5096–5102.
Article21. Gentry-Weeks CR, Karkhoff-Schweizer R, Pikis A, Estay M, Keith JM. Survival of Enterococcus faecalis in mouse peritoneal macrophages. Infect Immun. 1999. 67:2160–2165.
Article22. Okada H, Murakami S. Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med. 1998. 9:248–266.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of sonicates of Treponema denticola on osteoblast differentiation
- Sonicated extract of Treponema denticola impairs the lymphocyte proliferation
- Effect of Sonicated Extract of Treponema Denticola on Osteoclast Differentiation
- Characterization of Binding of Treponema denticola to Immobilized Fibrinogen using the Fluorescent Fatty Acid Labeling Method
- The Effect of Sonicated Extracts of Treponema Denticola and Treponema Lecithinolyticum on the Cytokine Secretion and Matrix Metalloproteinase Activation of Gingival Fibroblast