J Korean Acad Conserv Dent.
2002 Sep;27(5):473-478. 10.5395/JKACD.2002.27.5.473.
Sonicated extract of Treponema denticola impairs the lymphocyte proliferation
- Affiliations
-
- 1Department of Conservative Dentistry, Seoul National University, Korea.
- 2Department of Pathology, University of Pennsylvania, USA.
- KMID: 1987303
- DOI: http://doi.org/10.5395/JKACD.2002.27.5.473
Abstract
- No abstract available.
MeSH Terms
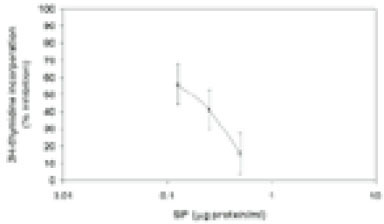
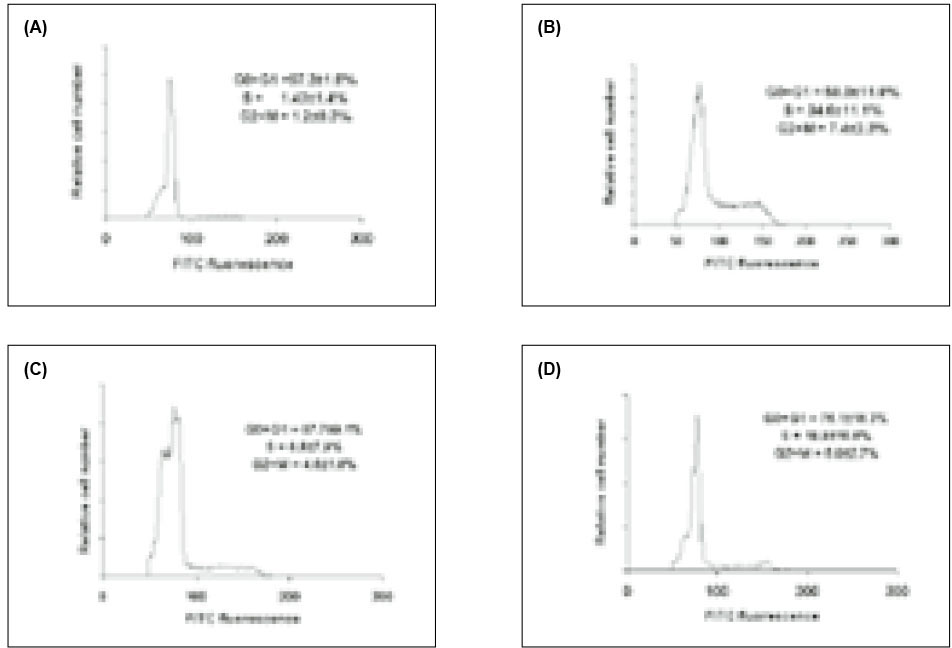
Figure
Reference
-
1. Listgarten MA. Genco RJ, Mergenhangen SE, editors. Colonization of subgingival areas by motile rods and spirochetes: clinical implication. Host-Parasite Interaction in Periodontal Diseases. 1982. Washington D.C: American Society of Microbiology;112.2. Thilo B, Baehni P, Holz J, Baume LJ. Distribution des bacteries dans les parties coronaire et apicale de dents a pulpe necrosee. SSO Schweiz Monatsschr Zahnheilkd. 1983. 93:335–350.3. Nair PNR. Light and electron microscopic studies of root canal flora and periapical lesions. J Endod. 1987. 13:29–39.
Article4. Siqueira JF, Rocas IN, Favieri A, Santos KRN. Detection of Treponema denticola in endodontics infections by 16S rRNA gene detected polymerase chain reaction. Oral Microbiol Immunol. 2000. 15:335–337.
Article5. Siqueira JF, Rocas IN, Oliveira JCM, Santos KRN. Detection of putative oral pathogens in acute periradicular abscesses by 16S rDNA-directed polymerase chain reaction. J Endod. 2001. 27:164–167.
Article6. Shenker BJ, Listgarten MA, Taichman NS. Suppression of human lymphocyte responses by oral spirochetes : A monocyte-dependent phenomenon. J Immunol. 1984. 132:2039–2045.7. Shenker BJ, Datar S. Fusobacterium nucleatum inhibits human T-cells by arresting cells in the mid G1 phase of the cell cycle. Infect Immun. 1995. 63:4830–4836.
Article8. Schumid I, Uittenbogaart CH, Giorgi JV. A gentle fixation and permeablization method for combined cell surface and intracellular staining with improved precision in DNA quantification. Cytometry. 1991. 12:279–285.
Article9. Shenker BJ, Mcarthur WP, Tsai CC. Immune suppression induced by Actinobacillus actinomycetemcomitans. I. Effects on human peripheral blood lymphocyte responses to mitogens and antigens. J Immunol. 1982. 128:148–154.10. Yoshida H, Sundqvist G, Bergenholtz G. Effect of sonicated material from Fusobacterium nucleatum on the functional capacity od accessory cells derived from dental pulp. Oral Microbiol Immunol. 1995. 10:208–212.
Article11. Holmgren J, Lindholm L, Lonnroth I. Interaction of cholera toxin and toxin derivatives with lymphocytes. I. binding properties and interference with lectin-induced cellular stimulation. J Exp Med. 1974. 139:801–819.12. Malakian AH, Schwab JH. Immunosuppressant from group A streptococci. Science. 1968. 159:880–881.
Article13. Hanna EE, Watson DW. Host-parasite relationships among group A streptococci. IV. Suppression of antibody response by streptococcal pyrogenic exotoxin. J Bacteriol. 1968. 90:14–21.
Article14. Floersheim GL, Hopff WH, Gasser M, Bucher K. Impairment of cell mediated immune response by Pseudomonas aeruginosa. Clin Exp Immunol. 1971. 9:241–247.15. Khansari N, Segre M, Segre D. Immunosuppression in murine malaria : a soluble immunosuppressive factor derived from Plasmodium berghei-infected blood. J Immunol. 1981. 127:1889–1893.16. Kauffman CA, Phair JP, Linneman CC, Schiff GM. Cell-mediated immunity in humans during viral infection. I. Effect of rubella on dermal hypersensitivity, PHA response and T lymphocytes numbers. Infect Immun. 1974. 10:212–215.
Article17. Olson GB, Dent PB, Rawls WE. Abnormalities of in vitro lymphocyte responses during rubella virus infections. J Exp Med. 1968. 128:47–68.
Article18. Kantzler GB, Lauteria SF, Cusumano CL, Lee JD, Ganguly R, Waldman RH. Immunosuppression during influenza virus infection. Infect Immun. 1974. 10:996–1002.
Article19. Engers HD, Louis JA, Zubler RH, Hirt B. Inhibition of Tx cell-mediated functions by MVM(i), a parvovirus closely related to minute virus of mice. J Immunol. 1981. 127:2280–2285.20. Cianciola LJ, Genco RJ, Peters MR, McKenna J, VanOss CJ. Defective polymorphonuclear leukocyte function in a human periodontal disease. Nature. 1977. 265:445–447.
Article21. Clark RA, Page RC, Wilde G. Defective neutrophil chemotaxis in juvenile periodontitis. Infect Immun. 1977. 18:694–700.
Article22. Van Dyke TE, Horoszewicz HH, Cianciola LJ, Genco RJ. Neutrophil chemotaxis dysfunction in human periodontitis. Infect Immun. 1980. 27:124–132.
Article23. Schwab JH. Suppression of the immune response by microorganisms. Bacteriol Rev. 1975. 39:121–143.
Article24. Shenker BJ, Tsai CC, Taichman NS. Suppression of lymphocyte responses by Actinobacillus actinomycetemcomitans. J Periodontal Res. 1982. 17:462.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Sonicated Extract of Treponema Denticola on Osteoclast Differentiation
- The effect of Treponema denticola immunoinhibitory protein on cytokine expression in T cells
- Effect of sonicates of Treponema denticola on osteoblast differentiation
- Characterization of Binding of Treponema denticola to Immobilized Fibrinogen using the Fluorescent Fatty Acid Labeling Method
- The Effect of Treponema Denticola and Treponema Lecithinolyticum on Periodontal Ligament Cells