Korean J Gynecol Oncol.
2008 Mar;19(1):68-74. 10.3802/kjgo.2008.19.1.68.
Effect of topical Paclitaxel using PEG/PLGA polymer on the animal model of cervical cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, The Catholic University of Korea College of Medicine, Seoul, Korea. jspark@catholic.ac.kr
- 2Department of Chemistry, Division of Nano Sciences, Ewha Womans University, Seoul, Korea.
- 3Laboratory of Infection and Immunology, Graduate School of Medicine, Korea University, Seoul, Korea.
- 4Department of Pathology, Samsung Cheil Hospital, Kwangdong University Medical College, Seoul, Korea.
- KMID: 1979503
- DOI: http://doi.org/10.3802/kjgo.2008.19.1.68
Abstract
-
OBJECTIVE
Paclitaxel is one of the most effective antineoplastic drugs. HPV-related cervical lesions have only managed with invasive procedure. Topical drug administration with temperature sensitive copolymer gels are useful approaches to clinical situation. In this study, we evaluated the activity of multiblock copolymers of PEG/PLA (poly(L-lactic acid)/polyethylene glycol) gels with paclitaxel (PTX) formulation administered by topical treatment to mice bearing human cervical cancer cell lines (HeLa).
METHODS
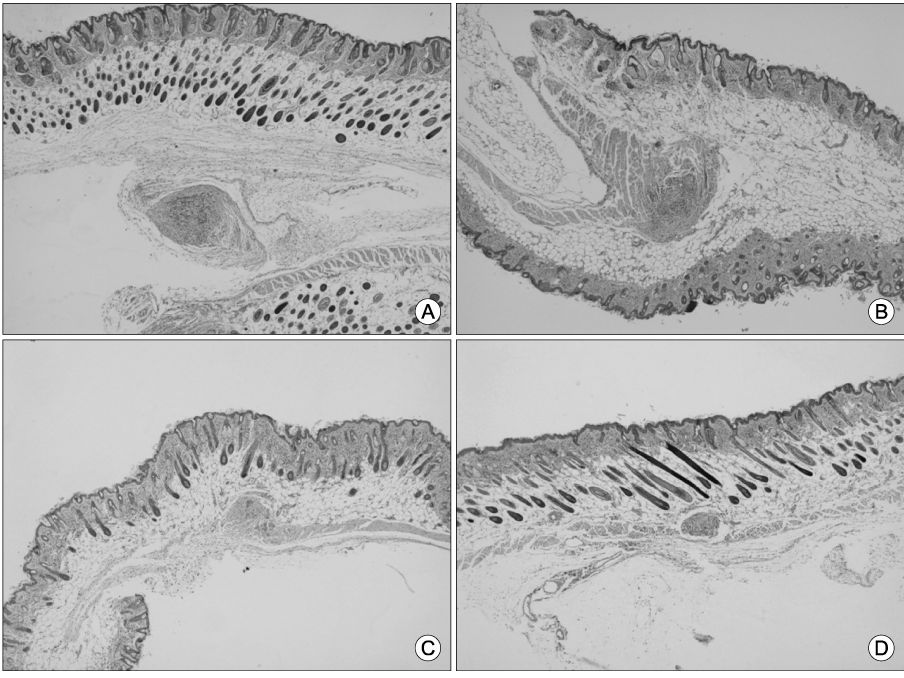
We have synthesized gels of PEG/PLLA (poly(L-lactic acid)/polyethylene glycol) multiblock copolymers containing Paclitaxel which have temperature-sensitivecharacteristics. This Paclitaxel-containg copolymers has the sol-gel-sol transition temperature at body temperature. The efficacy of PTX in PEG/PLA mutiblock copolymer micelle were conducted in HeLa-tumor bearing Balb/c Nu/Nu athymic mice at an equivalent paclitaxel dose of 10 mg/kg with 48 hr interval. The inhibition of tumor growth was evaluated after 8 days of treatment. Tumors were harvested at day 10 and stained with hematoxylin and eosine to measure tumor.
RESULTS
PTX-containing PEG/PLA mutiblock copolymer significantly decreased tumor growth at day 8, as measured by tumor size; ie, PEG/PLA mutiblock copolymer only goup ; 1.43+/-0.26 m versus intraperitoneal treatment of Paclitaxel : 0.75+/-0.07 mm and topical treatment of PTX-containing PEG/PLA copolymer containing Paclitaxel : 0.28 mm (Min; 0.1 mm-Maxu0.8 mm).
CONCLUSION
This demonstration that PTX-containing PEG/PLA mutiblock copolymer have a useful topical drug deliversy system carrying temperature sensitive characetersitics in HPV-related cervical lesions.
Keyword
MeSH Terms
-
Administration, Topical
Animals
Antineoplastic Agents
Body Temperature
Cell Line
Eosine Yellowish-(YS)
Gels
Hematoxylin
Humans
Lifting
Mice
Mice, Nude
Models, Animal
Paclitaxel
Polymers
Transition Temperature
Ursidae
Uterine Cervical Neoplasms
Antineoplastic Agents
Eosine Yellowish-(YS)
Gels
Hematoxylin
Paclitaxel
Polymers
Figure
Reference
-
1. Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006. 95:1459–1466.
Article2. Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002. 10:750–763.
Article3. Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005. 4:145–160.
Article4. Lee KH, Yim EK, Kim CJ, Namkoong SE, Um SJ, Park JS. Proteomic analysis of anti-cancer effects by paclitaxel treatment in cervical cancer cells. Gynecol Oncol. 2005. 9:45–53.
Article5. Working PK, Newman SS, Johnson J, Cornacoff JB. Harris JM, Zalipsky S, editors. Safety of poly(ethylene glycol) derivatives. Poly(ethylene glycol) Chemistry and Biological Applications. 1997. Washington, DC: ACS Books;45–54.6. Beletsi A, Panagi Z, Avgoustakis K. Biodistribution properties of nanoparticles based on mixtures of PLGA with PLGA-PEG diblock copolymers. Int J Pharm. 2005. 298:233–241.
Article7. Stivaktakis N, Nikou K, Panagi Z, Beletsi A, Leondiadis L, Avgoustakis K. Immune responses in mice of beta-galactosidase adsorbed or encapsulated in poly (lactic acid) and poly (lactic-co-glycolic acid) microspheres. J Biomed Mater Res A. 2005. 73:332–338.8. Parness J, Horwitz SB. Taxol binds to polymerized tubulin in vitro. J Cell Biol. 1981. 91:479–487.
Article9. Manfredi JJ, Parness J, Horwitz SB. Taxol binds to cellular microtubules. J Cell Biol. 1982. 94:688–696.
Article10. Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001. 37:1590–1598.11. Klooer JS, den Bakker MA, Gelderblom H, van Meerbeeck JP. Fatal outcome of a hypersensitivity reactions to paclitaxel: A critical review of premedication regimens. Br J Cancer. 2004. 90:304–305.12. Langer CJ. CT-2103: A novel macromolecular taxane with potential advantages compared with conventional taxanes. Clin Lung Cancer. 2004. 6:S85–S88.
Article13. Sabbatini P, Aghajanian C, Dizon D, Anderson S, Dupont J, Brown JV, et al. Phase II study of CT-2103 in patients with recurrent ovarian, fallopian tube, or primary peritoneal carcinoma. J Clin Oncol. 2004. 22:4523–4531.14. Kudelka AP, Verschraegen CF, Loyer E, Wallace S, Gershenson DM, Han J, et al. Preliminary report of a phase I study of escalating dose PG-paclitaxel (CT-2103) and fixed dose cisplatin in patients with solid tumors. Proc Am Soc Clin Oncol. 2002. 2146.15. Socinski M. XYOTAX in NSCLC and other solid tumors. Emerging evidence on biological sex differences: Is genderspecific therapy warranted? 2005. In : Chemotherapy Foundation XXIII symposium innovative cancer therapy for tomorrow; November; Mount Sinai.16. Ross H, Bonomi P, Langer P, O'Brien M, O'Byrne K, Paz-Ares L, et al. Effect of gender on outcome in two randomized phase III trials of paclitaxel poliglumex (PPX) in chemonaive pts with advanced NSCLC and poor performance status (PS2). 2006. In : ASCO Annual Meeting; 7039.17. Kremer M, Judd J, Rifkin B, Auszmann J, Oursler MJ. Estrogen modulation of osteoclast lysosomalenzyme secretion. J Cellular Biochem. 1995. 57:271–279.18. Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994. 263:1600–1603.
Article19. Verrecchia T, Huve P, Bazile D, Veillard M, Spenlehauer G, Couvreur P. Adsorption/desorption of human serum albumin at the surface of poly(lactic acid) nanoparticles prepared by a solvent evaporation process. J Biomed Mater Res. 1993. 27:1019–1028.
Article20. Peppas NA, De Ascentiis A, Bauerle JM. Mucoadhesive PEG-tethered microparticulate systems for gastrointestinal and vaginal drug delivery. Proceed Int Symp Control Release Bioact Mater. 1996. 214–215.21. Martini L, Attwood D, Collet JH, D'Emanuele A. The bioadhesive properties of a triblock copolymer of ocaprolactone and ethylene oxide. Int J Pharm. 1995. 113:223–229.22. Tobio M, Gref R, Sanchez A, Langer R, Alonso MJ. Stealth PEG-PLA nanoparticles as protein carriers for nasal administration. Pharm Res. 1998. 15:270–275.23. Tobio M, Sanchez A, Vila A, Soriano I I, Evora C, Vila-Jato JL, et al. The role of PEGon the stability in digestive fluids and in vivo fate of PEG-PLA nanoparticles following oral administration. Colloids Surf B Biointerfaces. 2000. 18:315–323.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study of Drug Content and Cell Cytotoxicity of Paclitaxel-eluting Stents Coated with Various Biopolymer
- Clusterin expression and paclitaxel resistance in cervical cancer cell lines
- The Histopathologic Findings after Insertion of Biodegradible Polymer Sheet Made of PGA and PLGA/5-FU with Filtering Surgery in the Rabbit
- Sheet Form Transplantation of Cultured Retinal Pigment Epithelial Cells with Poly lactic-co-glycolic acid
- Development of a therapeutic method in the HPV-related cervical lesion using pH/temperature sensitive polymer spray formulation