Tuberc Respir Dis.
2007 Apr;62(4):276-283. 10.4046/trd.2007.62.4.276.
Clinical Value of a Desktop Spirometer (HI-801) for Spirometry Screening
- Affiliations
-
- 1Department of Pulmonary and Critical Care Medicine, Kyung Hee University College of Medicine, Seoul, Korea.
- 2Kyung Hee Medical Center, Seoul, Korea.
- 3East-West Neomedical Center, Seoul, Korea. honglung@chollian.net
- KMID: 1970227
- DOI: http://doi.org/10.4046/trd.2007.62.4.276
Abstract
-
BACKGROUND: A national health care initiative recommends routine spirometry screening of all smokers over age 45 or patients with respiratory symptoms. In response to the recommendation, new, simple, and inexpensive desktop spirometers for the purpose of promoting widespread spirometric screening were marketed. The performance of these spirometers was evaluated in vivo testing with healthy subjects. However, the clinical setting allows spirometric assessment of various pathologic combinations of flow and volume.
OBJECTIVE
The aim of this study was to compare the accuracy of a desktop spirometer to a standard laboratory spirometer, in a clinical setting with?pathologic pulmonary function. METHOD: In a health check-up center, where screening pulmonary funct test was performed using the HI-801 spirometer. Subjects who revealed the ventilation defect in screening spirometry, performed the spirometry again using the?standard Vmax spectra 22d spirometer in a tertiary care hospital pulmonary function laboratory. Pulmonary function test with both spirometer was performed according to the guidelines of the American Thoracic Society.
RESULTS
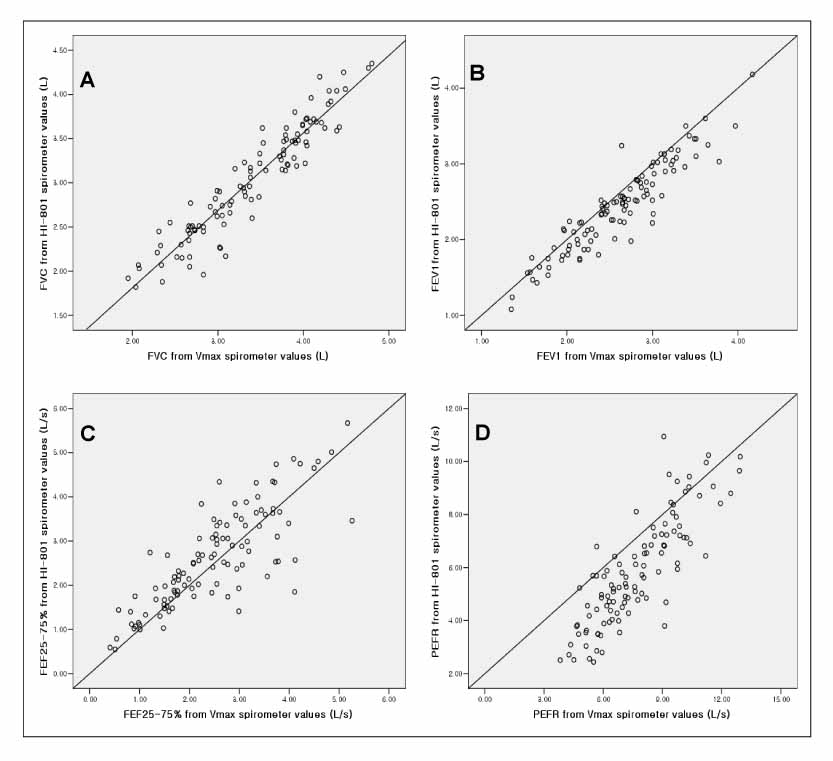
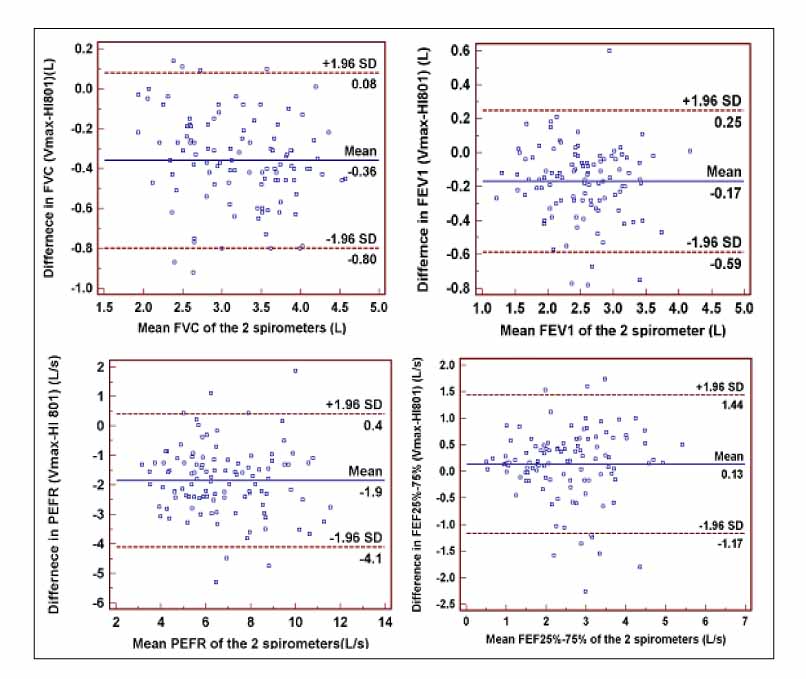
109 patients were enrolled. Pulmonary function measurements (FVC, FEV1, PEFR, FEF25%-75%) from the HI-801 correlated closely (r=0.94, 0.93, 0.81, 0.84, respectively) with those performed with the Vmax spectra 22d?and showed the good limits of agreement and differences between the 2 devices; FVC +0.35 L, FEV1 +0.16 L, PEFR +1.85 L/s, FEF25%-75% -0.13 L/s. With the exception of FEV1, FEF25%-75%, these differences were significant(p<0.05) but small.
Conclusion
The HI-801 spirometer is comparable to the standard laboratory spirometer, Vmax spectra 22d, with high accurary for FEV1 and FVC and?acceptable differences for clinical use.
MeSH Terms
Figure
Reference
-
1. Ferguson GT, Enright PL, Buist AS, Higgins MW. Office spirometry for lung health assessment in adults: a consensus statement from the National Lung Health Education Program. Chest. 2000. 117:1146–1161.2. The World Health Report. 2002: reducing risks, promoting healthy life. 2002. Geneva, Switzerland: World Health Organization;Available from: http://www.who.int/whr/2002.3. Wise RA, Kanner RE, Lindgren P, Connett JE, Altose MD, Enright PL, et al. The effect of smoking intervention and an inhaled bronchodilator on airways reactivity in COPD: the Lung Health Study. Chest. 2003. 124:449–458.4. Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. JAMA. 1994. 272:1497–1505.5. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005. 26:319–338.6. American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995. 152:1107–1136.7. Korhonen H, Remes ST, Kannisto S. Hand-held turbine spirometer: agreement with the conventional spirometer at baseline and after exercise. Pediatr Allergy Immunol. 2005. 16:254–257.8. Maree DM, Videler EA, Hallauer M, Pieper CH, Bolliger CT. Comparison of a new desktop spirometer (Diagnosa) with a laboratory spirometer. Respiration. 2001. 68:400–404.9. Swart F, Schuurmans MM, Heydenreich JC, Pieper CH, Bolliger CT. Comparison of a new desktop spirometer (Spirospec) with a laboratory spirometer in a respiratory out-patient clinic. Respir Care. 2003. 48:591–595.10. Rebuck DA, Hanania NA, D'Urzo AD, Chapman KR. The accuracy of a handheld portable spirometer. Chest. 1996. 109:152–157.11. HI-801® Operator's Manual. Version 2.7. 2004. Tokyo, Japan: CHEST M.I., Inc.12. Vmax® Operator's Manual. 2003. Yorba Linda, CA: Sensor Medics Corporation.13. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986. 1:307–310.14. Schoh RJ, Fero LJ, Shapiro H, Aslor JP, Kaelin OJ, Rollins DR, Petty TL. Performance of a new screening spirometer at a community health fair. Respir Care. 2002. 47:1150–1157.15. Robert ED, Katherine LV, Jennifer C, Shawn DA. Spirometry in primary care setting: Influence on clinical diagnosis and management of airflow obstruction. Chest. 2005. 128:2443–2447.16. Izbicki G, Abboud S, Jordan P, Perruchoud AP, Bolliger CT. A comparison of a new transtelephonic portable spirometer with a laboratory spirometer. Eur Respir J. 1999. 14:209–213.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pharyngeal reperforation following incentive spirometry: A case report
- The Effects of Deep Breathing Methods on Pulmonary Ventilatory Function of Pneumothorax Patients undergoing a Thoracotomy
- A Study of the Usefluness of Forced Expiratory Spirometry as a Preoperative Laboratory Screeing in the Elderly Patient
- Changes of Pulmonary Disability Grades according to the Spirometry Reference Equations
- Spirometry, a useful method for detecting upper airway tumor