Korean J Urol.
2007 Jan;48(1):87-93. 10.4111/kju.2007.48.1.87.
Temporary Opening of the Testis-blood Barrier by Triolein Fat Emulsion
- Affiliations
-
- 1Department of Urology, College of Medicine, Pusan National University, Busan, Korea.
- 2Department of Radiology, College of Medicine, Pusan National University, Busan, Korea. hakjink@pusan.ac.kr
- KMID: 1915028
- DOI: http://doi.org/10.4111/kju.2007.48.1.87
Abstract
-
PURPOSE: Although the purpose of the blood-testis barrier (BTB) is to protect germ cells from harmful influences, it also impedes the delivery of chemotherapeutic agents to the testis. This study was undertaken to determine whether a triolein emulsion could transiently alter the permeability of the BTB in cats.
MATERIALS AND METHODS
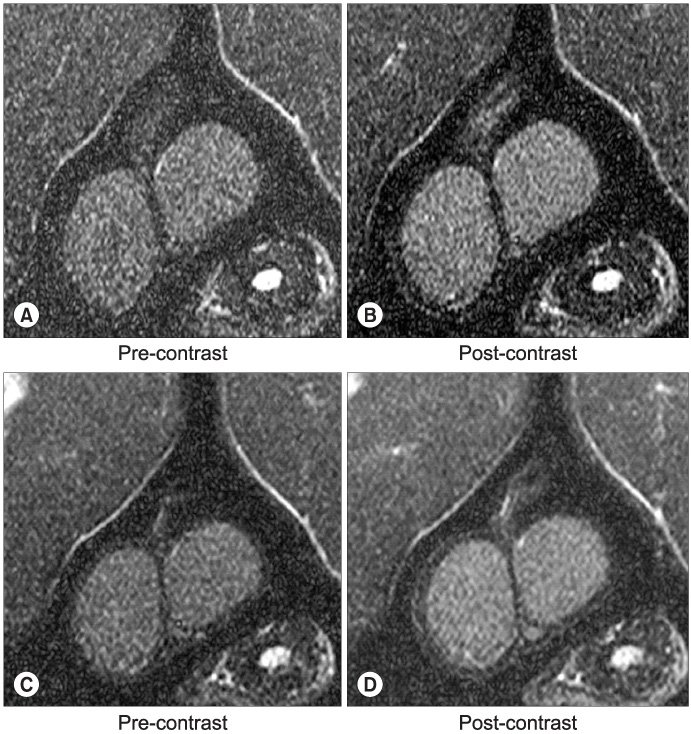
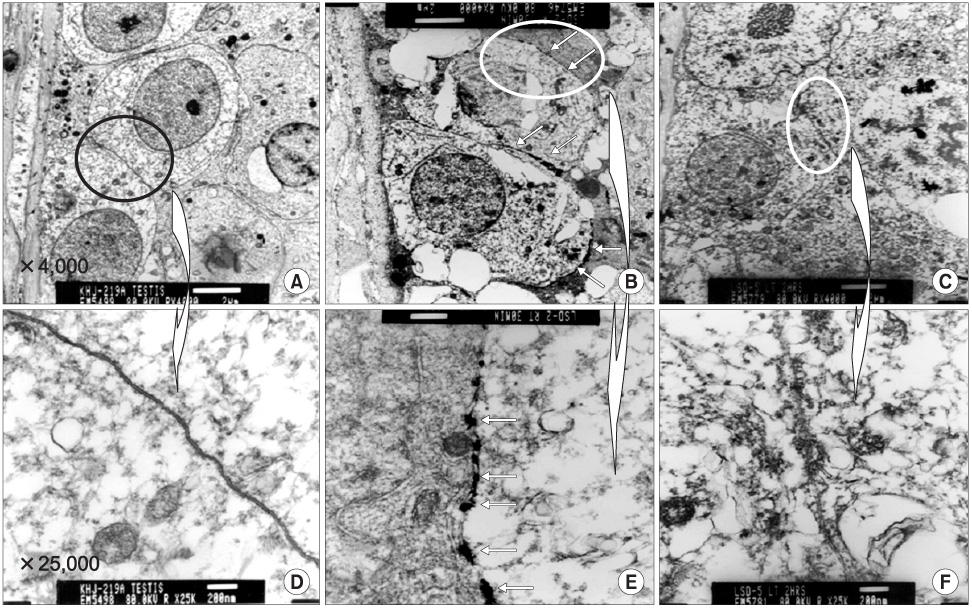
An emulsion of 0.05ml triolein in 20ml of saline or just 20ml of normal saline, as the control, were infused into the testicular arteries in 18 and 15 cats, respectively (embolic and control group). Pre- and post-contrast magnetic resonance images (MRIs) were obtained 30 minutes and 2 hours after embolization. Qualitative and quantitative analyses of the MRIs were performed via the presence and degree of contrast enhancement and the contrast enhancement ratios (CERs), respectively. An electron microscopy (EM) study was subsequently performed, using a lanthanum tracer, to correlate with the MRI results.
RESULTS
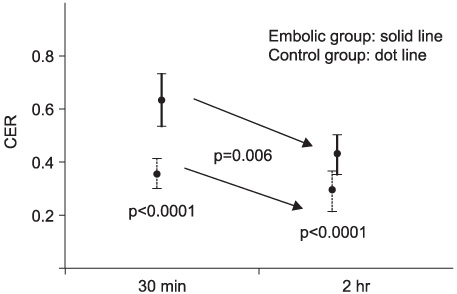
Contrast enhancement of the testis was observed in both groups and at both time points, but was more prominent in the embolic group. The CERs in the embolic group were significantly higher than those in the control group (p=0.0001). In each group, the CERs at 2 hours were significantly lower than those at 30 minutes (p=0.006). In the EM study, the entry of lanthanum was markedly increased at 30 mins, but recovered at 2 hours after embolization compared to the control.
CONCLUSIONS
Intra-arterial infusion of triolein emulsion transiently increased the permeability of the BTB. This result may be useful in future studies for a chemotherapy delivery system to the testis.
Keyword
MeSH Terms
Figure
Reference
-
1. Bart J, Groen HJ, van der Graaf WT, Hollema H, Hendrikse NH, Vaalburg W, et al. An oncological view on the bloodtestis barrier. Lancet Oncol. 2002. 3:357–363.2. Kamps WA, Bokkerink JP, Hahlen K, Hermans J, Riehm H, Gardner H, et al. Intensive treatment of children with acute lymphoblastic leukemia according to ALL-BFM-86 without cranial radiotherapy: results of Dutch Childhood Leukemia Study Group Protocol ALL-7 (1988-1991). Blood. 1999. 94:1226–1236.3. Miniero R, Saracco P, Pastore G, Zurlo MG, Terracini B, Rosso P, et al. Relapse after first cessation of therapy in childhood acute lymphoblastic leukemia: a 10-year follow-up study. Italian Association of Pediatric Hematology-Oncology (AIEOP). Med Pediatr Oncol. 1995. 24:71–76.4. Touroutoglou N, Dimopoulos MA, Younes A, Hess M, Pugh W, Cox J, et al. Testicular lymphoma: late relapses and poor outcome despite doxorubicin-based therapy. J Clin Oncol. 1995. 13:1361–1367.5. Tondini C, Ferreri AJ, Siracusano L, Valagussa P, Giardini R, Rampinelli I, et al. Diffuse large-cell lymphoma of the testis. J Clin Oncol. 1999. 17:2854–2858.6. Kroll RA, Neuwelt EA. Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998. 42:1083–1099.7. Kim HJ, Lee CH, Kim HG, Lee SD, Son SM, Kim YW, et al. Reversible MR changes in the cat brain after cerebral fat embolism induced by triolein emulsion. AJNR Am J Neuroradiol. 2004. 25:958–963.8. Sun EL, Gondos B. Formation of the blood-testis barrier in the rabbit. Cell Tissue Res. 1986. 243:575–578.9. Forssmann WG, Ito S, Weihe E, Aoki A, Dym M, Fawcett DW. An improved perfusion fixation method for the testis. Anat Rec. 1977. 188:307–314.10. Runge VM, Price AC, Wehr CJ, Atkinson JB, Tweedle MF. Contrast enhanced MRI: evaluation of a canine model of osmotic blood-brain barrier disruption. Invest Radiol. 1985. 20:830–844.11. Farghali H, Williams DS, Simplaceanu E, Ho C, Van Thiel DH. An evaluation of the integrity of the blood-testis barrier by magnetic resonance imaging. Magn Reson Med. 1991. 22:81–87.12. Kim HJ, Lee CH, Lee SH, Moon TY. Early development of vasogenic edema in experimental cerebral fat embolism in cat. Invest Radiol. 2001. 36:460–469.13. Eng F, Wiebe JP, Alima LH. Long-term alterations in the permeability of the blood-testis barrier following a single intratesticular injection of dilute aqueous glycerol. J Androl. 1994. 15:311–317.14. Wiebe JP, Kowalik A, Gallardi RL, Egeler O, Clubb BH. Glycerol disrupts tight junction-associated actin microfilaments, occludin, and microtubules in Sertoli cells. J Androl. 2000. 21:625–635.15. Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartment of the seminiferous epithelium. Biol Reprod. 1970. 3:308–326.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Experimental Model for Research on the Blood-Ocular Barrier
- The Steroid Effect on the Blood-Ocular Barrier Change Induced by Triolein Emulsion as seen on Contrast-Enhanced MR Images
- Characteristics of Focused Ultrasound Mediated Blood-Brain Barrier Opening in Magnetic Resonance Images
- Experimental Study on Fat Absorption (I131-Triolein) from the Parasite Infected Intestine
- Blood-Brain Barrier Experiments with Clinical Magnetic Resonance Imaging and an Immunohistochemical Study