J Korean Neurosurg Soc.
2023 Mar;66(2):172-182. 10.3340/jkns.2022.0236.
Characteristics of Focused Ultrasound Mediated Blood-Brain Barrier Opening in Magnetic Resonance Images
- Affiliations
-
- 1Brain Research Institute, Department of Neurosurgery, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2539878
- DOI: http://doi.org/10.3340/jkns.2022.0236
Abstract
Objective
: The blood-brain barrier (BBB) is an obstacle for molecules to pass through from blood to the brain. Focused ultrasound is a new method which temporarily opens the BBB, which makes pharmaceutical delivery or removal of neurodegenerative proteins possible. This study was demonstrated to review our BBB opening procedure with magnetic resonance guided images and find specific patterns in the BBB opening.
Methods
: In this study, we reviewed the procedures and results of two clinical studies on BBB opening using focused ultrasound regarding its safety and clinical efficacy. Magnetic resonance images were also reviewed to discover any specific findings.
Results
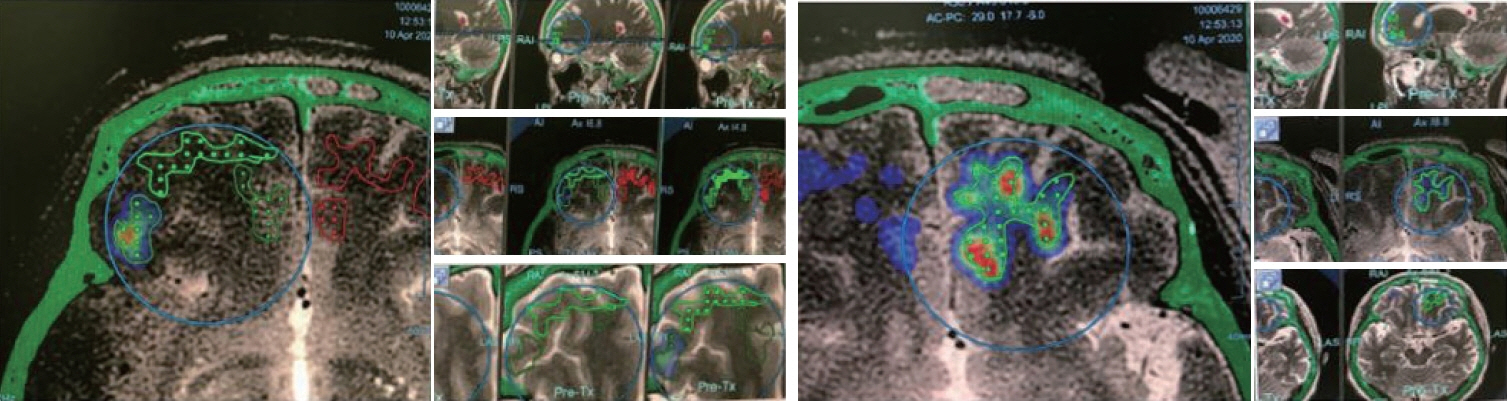
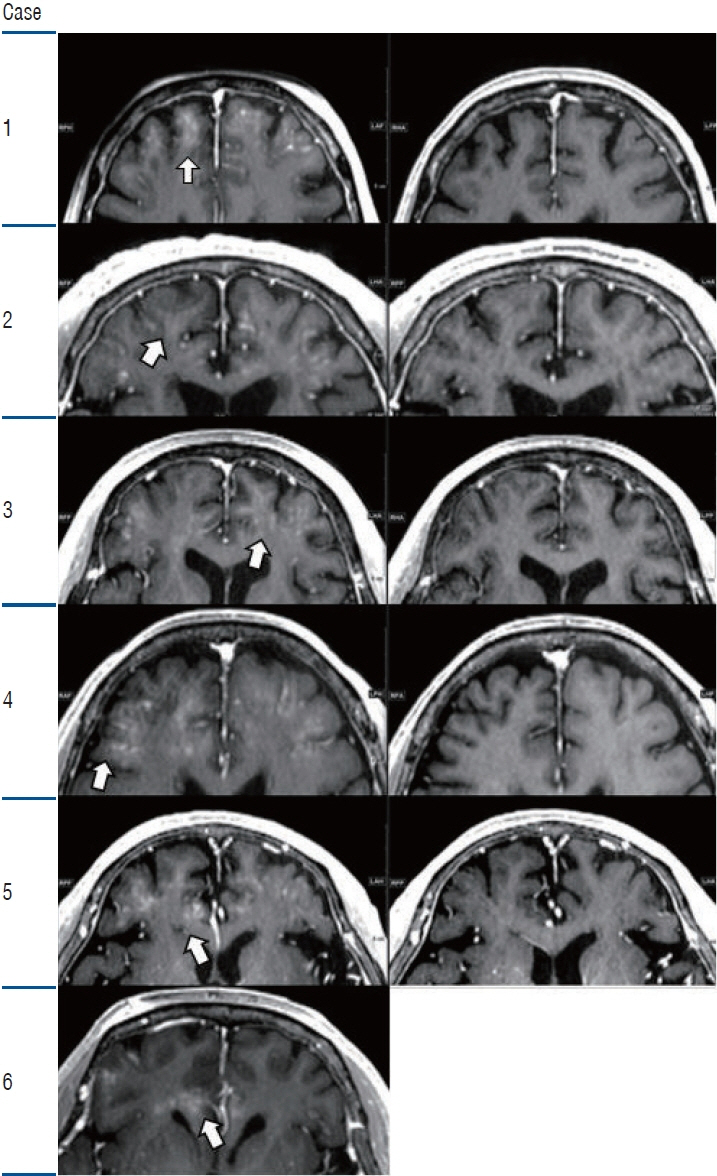
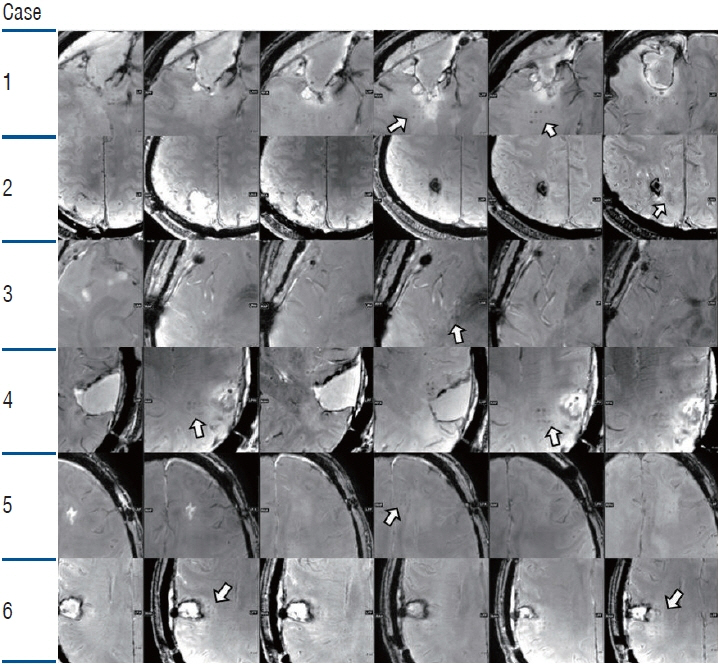
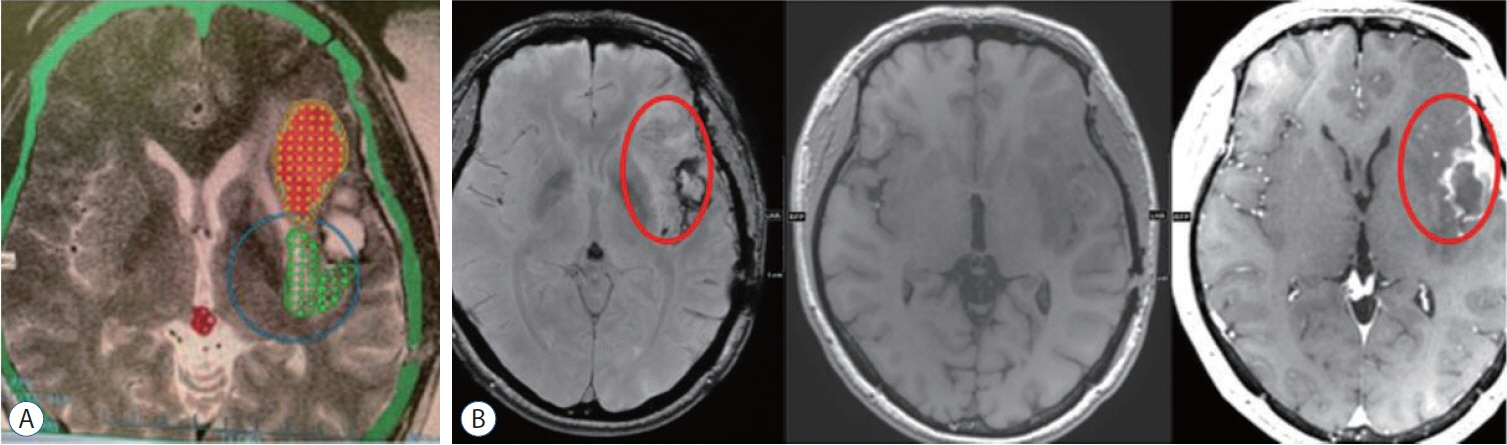
: Two clinical trials showed clinical benefits. All clinical trials demonstrated safe BBB opening, with no specific side effects. Magnetic resonance imaging showed temporary T1 contrast enhancement in the sonication area, verifying the BBB opening. Several low-signal intensity spots were observed in the T2 susceptibility-weighted angiography images, which were also reversible and temporary. Although these spots can be considered as microbleeding, evidence suggests these are not ordinary microbleeding but an indicator for adequate BBB opening.
Conclusion
: Magnetic resonance images proved safe and efficient BBB opening in humans, using focused ultrasound.
Figure
Reference
-
References
1. Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 7:41–53. 2006.2. Anastasiadis P, Gandhi D, Guo Y, Ahmed AK, Bentzen SM, Arvanitis C, et al. Localized blood-brain barrier opening in infiltrating gliomas with MRI-guided acoustic emissions-controlled focused ultrasound. Proc Natl Acad Sci U S A. 118:e2103280118. 2021.3. Aryal M, Vykhodtseva N, Zhang YZ, Park J, McDannold N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model. J Control Release. 169:103–111. 2013.4. Bakay L, Ballantine HT Jr, Hueter TF, Sosa D. Ultrasonically produced changes in the blood-brain barrier. AMA Arch Neurol Psychiatry. 76:457–467. 1956.5. Burgess A, Shah K, Hough O, Hynynen K. Focused ultrasound-mediated drug delivery through the blood-brain barrier. Expert Rev Neurother. 15:477–491. 2015.6. Chang KW, Jung HH, Chang JW. Magnetic resonance-guided focused ultrasound surgery for obsessive-compulsive disorders: potential for use as a novel ablative surgical technique. Front Psychiatry. 12:640832. 2021.7. Chang WS, Jung HH, Kweon EJ, Zadicario E, Rachmilevitch I, Chang JW. Unilateral magnetic resonance guided focused ultrasound thalamotomy for essential tremor: practices and clinicoradiological outcomes. J Neurol Neurosurg Psychiatry. 86:257–264. 2015.8. Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 7:a020412. 2015.9. Docampo J, Gonzalez N, Bravo F, Sarroca D, Morales C, Bruno C. Susceptibility-weighted angiography of intracranial blood products and calcifications compared to gradient echo sequence. Neuroradiol J. 26:493–500. 2013.10. Elias WJ, Lipsman N, Ondo WG, Ghanouni P, Kim YG, Lee W, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 375:730–739. 2016.11. Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, Schmidt R, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 20:637–642. 1999.12. Haller S, Vernooij MW, Kuijer JPA, Larsson EM, Jäger HR, Barkhof F. Cerebral microbleeds: imaging and clinical significance. Radiology. 287:11–28. 2018.13. Hersh DS, Wadajkar AS, Roberts N, Perez JG, Connolly NP, Frenkel V, et al. Evolving drug delivery strategies to overcome the blood brain barrier. Curr Pharm Des. 22:1177–1193. 2016.14. Hynynen K. MRI-guided focused ultrasound treatments. Ultrasonics. 50:221–229. 2010.15. Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 220:640–646. 2001.16. Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Non-invasive opening of BBB by focused ultrasound. Acta Neurochir Suppl. 86:555–558. 2003.17. Jordão JF, Ayala-Grosso CA, Markham K, Huang Y, Chopra R, McLaurin J, et al. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer’s disease. PLoS One. 5:e10549. 2010.18. Jung HH, Kim SJ, Roh D, Chang JG, Chang WS, Kweon EJ, et al. Bilateral thermal capsulotomy with MR-guided focused ultrasound for patients with treatment-refractory obsessive-compulsive disorder: a proof-ofconcept study. Mol Psychiatry. 20:1205–1211. 2015.19. Karakatsani ME, Blesa J, Konofagou EE. Blood-brain barrier opening with focused ultrasound in experimental models of Parkinson’s disease. Mov Disord. 34:1252–1261. 2019.20. Kim SJ, Roh D, Jung HH, Chang WS, Kim CH, Chang JW. A study of novel bilateral thermal capsulotomy with focused ultrasound for treatment-refractory obsessive-compulsive disorder: 2-year follow-up. J Psychiatry Neurosci. 43:327–337. 2018.21. Lipsman N, Meng Y, Bethune AJ, Huang Y, Lam B, Masellis M, et al. Blood-brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat Commun. 9:2336. 2018.22. Liu HL, Hua MY, Chen PY, Chu PC, Pan CH, Yang HW, et al. Bloodbrain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology. 255:415–425. 2010.23. Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, Ironside S, et al. Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci Rep. 9:321. 2019.24. Martínez-Fernández R, Rodríguez-Rojas R, Del Álamo M, Hernández-Fernández F, Pineda-Pardo JA, Dileone M, et al. Focused ultrasound subthalamotomy in patients with asymmetric Parkinson’s disease: a pilot study. Lancet Neurol. 17:54–63. 2018.25. McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 72:3652–3663. 2012.26. McDannold N, Clement GT, Black P, Jolesz F, Hynynen K. Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery. 66:323–332. discussion 332. 2010.27. McDannold N, Vykhodtseva N, Hynynen K. Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood-brain barrier disruption. Ultrasound Med Biol. 34:930–937. 2008.28. McDannold N, Zhang Y, Supko JG, Power C, Sun T, Peng C, et al. Acoustic feedback enables safe and reliable carboplatin delivery across the blood-brain barrier with a clinical focused ultrasound system and improves survival in a rat glioma model. Theranostics. 9:6284–6299. 2019.29. McMahon D, Hynynen K. Acute inflammatory response following increased blood-brain barrier permeability induced by focused ultrasound is dependent on microbubble dose. Theranostics. 7:3989–4000. 2017.30. Meng Y, Abrahao A, Heyn CC, Bethune AJ, Huang Y, Pople CB, et al. Glymphatics visualization after focused ultrasound-induced blood-brain barrier opening in humans. Ann Neurol. 86:975–980. 2019.31. Na YC, Chang WS, Jung HH, Kweon EJ, Chang JW. Unilateral magnetic resonance-guided focused ultrasound pallidotomy for Parkinson disease. Neurology. 85:549–551. 2015.32. Nisbet RM, Van der Jeugd A, Leinenga G, Evans HT, Janowicz PW, Götz J. Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model. Brain. 140:1220–1230. 2017.33. Pandit R, Chen L, Götz J. The blood-brain barrier: physiology and strategies for drug delivery. Adv Drug Deliv Rev. 165-166:1–14. 2020.34. Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2:3–14. 2005.35. Pardridge WM. CSF, blood-brain barrier, and brain drug delivery. Expert Opin Drug Deliv. 13:963–975. 2016.36. Park SH, Baik K, Jeon S, Chang WS, Ye BS, Chang JW. Extensive frontal focused ultrasound mediated blood-brain barrier opening for the treatment of Alzheimer’s disease: a proof-of-concept study. Transl Neurodegener. 10:44. 2021.37. Park SH, Kim MJ, Jung HH, Chang WS, Choi HS, Rachmilevitch I, et al. One-year outcome of multiple blood-brain barrier disruptions with temozolomide for the treatment of glioblastoma. Front Oncol. 10:1663. 2020.38. Park SH, Kim MJ, Jung HH, Chang WS, Choi HS, Rachmilevitch I, et al. Safety and feasibility of multiple blood-brain barrier disruptions for the treatment of glioblastoma in patients undergoing standard adjuvant chemotherapy. J Neurosurg. 134:475–483. 2020.39. Rezai AR, Ranjan M, D’Haese PF, Haut MW, Carpenter J, Najib U, et al. Noninvasive hippocampal blood-brain barrier opening in Alzheimer’s disease with focused ultrasound. Proc Natl Acad Sci U S A. 117:9180–9182. 2020.40. Sheikov N, McDannold N, Sharma S, Hynynen K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med Biol. 34:1093–1104. 2008.41. Shin J, Kong C, Cho JS, Lee J, Koh CS, Yoon MS, et al. Focused ultrasound-mediated noninvasive blood-brain barrier modulation: preclinical examination of efficacy and safety in various sonication parameters. Neurosurg Focus. 44:E15. 2018.42. Shin J, Kong C, Lee J, Choi BY, Sim J, Koh CS, et al. Focused ultrasound-induced blood-brain barrier opening improves adult hippocampal neurogenesis and cognitive function in a cholinergic degeneration dementia rat model. Alzheimers Res Ther. 11:110. 2019.43. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005.44. Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 121:901–907. 2007.45. Wang DN, Hou XW, Yang BW, Lin Y, Shi JP, Wang N. Quantity of cerebral microbleeds, antiplatelet therapy, and intracerebral hemorrhage outcomes: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 24:2728–2737. 2015.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Magnetic Resonance-Guided Focused Ultrasound : Current Status and Future Perspectives in Thermal Ablation and Blood-Brain Barrier Opening
- Magnetic Resonance-Guided Focused Ultrasound in Neurosurgery: Taking Lessons from the Past to Inform the Future
- Biological effects of blood–brain barrier disruption using a focused ultrasound
- A pilot clinical study of low-intensity transcranial focused ultrasound in Alzheimer’s disease
- Anatomic illustrations of Cranial Ultrasound Images in the Neonate: Objective Analysis of the Oblique Sonographic Scans using MRI and a Reconstruction Program