Korean Circ J.
2010 Oct;40(10):491-498. 10.4070/kcj.2010.40.10.491.
High Lipoprotein(a) Levels are Associated With Long-Term Adverse Outcomes in Acute Myocardial Infarction Patients in High Killip Classes
- Affiliations
-
- 1Heart Research Center of Chonnam National University Hospital, Cardiovascular Research Institute of Chonnam National University, Gwangju, Korea. myungho@chollian.net
- KMID: 1826137
- DOI: http://doi.org/10.4070/kcj.2010.40.10.491
Abstract
- BACKGROUND AND OBJECTIVES
An elevated concentration of lipoprotein(a) {Lp(a)} is associated with an increased prevalence and increased severity of coronary artery disease. However, the relationship between Lp(a) levels and outcomes after acute myocardial infarction (AMI) is unclear.
SUBJECTS AND METHODS
Between October 2005 and June 2007, we measured serum Lp(a) levels in 832 consecutive AMI patients (age, 62.8+/-12.4 years, 600 men) on admission. They were divided into tertiles according to their serum Lp(a) levels {Tertile 1 (n=276), Lp(a)<13.8 mg/dL; Tertile 2 (n=279), Lp(a)=13.8-30.6 mg/dL; Tertile 3 (n=277), Lp(a)>30.6 mg/dL}.
RESULTS
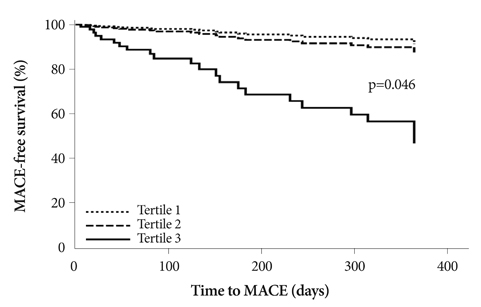
There were no differences in baseline clinical characteristics among Tertiles 1, 2, and 3, except for proportions of Killip class III-IV patients (5.8% vs. 10.0% vs. 18.8%, respectively, p<0.001). There were significant differences in left ventricular ejection fractions (57.3+/-11.4% vs. 55.9+/-12.3% vs. 53.1+/-13.1%, p<0.001). Among the laboratory findings, there were significant differences in total cholesterol (173.3+/-37.2 vs. 183.5+/-38.9 vs. 185.3+/-43.8 mg/dL, p=0.001), low density lipoprotein-cholesterol (111.3+/-34.3 vs. 122.9+/-34.7 vs. 123.3+/-39.4 mg/dL, p<0.001), apolipoprotein B (92.8+/-25.4 vs. 100.8+/-26.0 vs. 101.9+/-28.8 mg/dL, p<0.001), and amino-terminal pro-brain natriuretic peptide levels (1805.2+/-4343.3 vs. 2607.9+/-5216.3 vs. 3981.5+/-7689.7 pg/mL, p<0.001). After adjusting for multiple variables in the high Killip class (III-IV) subgroup, the risk estimate for major adverse cardiovascular events (MACE) at 1-year follow-up was significantly higher in Tertile 3 than in Tertiles 1 or 2 (hazard ratio 6.723, 95% confidence interval 1.037-43.593, p=0.046).
CONCLUSION
In patients in high Killip classes, high serum levels of Lp(a) were significantly associated with long-term adverse outcomes after AMI.
Keyword
MeSH Terms
Figure
Reference
-
1. Lee CK. Lipoprotein(a), Lp(a). Korean Circ J. 1993. 23:631–633.2. Hajjar KA, Gavish D, Breslow JL, Nachman RL. Lipoprotein (a) modulation of endothelial surface fibrinolysis and its potential role in atherosclerosis. Nature. 1989. 339:303–305.3. Harpel PC, Gordon BR, Parker TS. Plasmin catalyzes binding of lipoprotein (a) to immobilized fibrinogen and fibrin. Proc Natl Acad Sci U S A. 1989. 86:3847–3851.4. Harpel PC, Chang VT, Borth W. Homocysteine and other sulfhydryl compounds enhance the binding of lipoprotein (a) to fibrin: a potential biochemical link between thrombosis, atherogenesis, and sulfhydryl compound metabolism. Proc Natl Acad Sci U S A. 1992. 89:10193–10197.5. Park SH, Shin GJ. Lipoprotein (a) as a risk factor for coronary heart disease: whether related with NIDDM or not. Korean Circ J. 1996. 26:507–513.6. Jürgens G, Taddei-Peters WC, Költringer P, et al. Lipoprotein (a) serum concentration and apolipoprotein (a) phenotype correlate with severity and presence of ischemic cerebrovascular disease. Stroke. 1995. 26:1841–1848.7. Danesh J, Collins R, Peto R. Lipoprotein (a) and coronary heart disease: meta-analysis of prospective studies. Circulation. 2000. 102:1082–1085.8. Kamstrup PR, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Extreme lipoprotein (a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008. 117:176–184.9. Foody JM, Milberg JA, Pearce GL, Sprecher DL. Lipoprotein (a) associated with coronary artery disease in older women: age and gender analysis. Atherosclerosis. 2000. 153:445–451.10. Jenner JL, Ordovas JM, Lamonfava S, et al. Effects of age, sex, and menopausal status on plasma lipoprotein (A) levels: the Framingham Offspring Study. Circulation. 1993. 87:1135–1141.11. Seman LJ, Breckenridge WC. Isolation and partial characterization of apolipoprotein (a) from human lipoprotein (a). Biochem Cell Biol. 1986. 64:999–1009.12. Carlson LA, Hamsten A, Asplund A. Pronounced lowering of serum levels of lipoprotein Lp(a) in hyperlipemic subjects treated with nicotinic-acid. J Intern Med. 1989. 226:271–276.13. Morishita R, Ishii J, Kusumi Y, et al. Association of serum oxidized lipoprotein (a) concentration with coronary artery disease: potential role of oxidized lipoprotein (a) in the vascular wall. J Atheroscler Thromb. 2009. 16:410–418.14. Kostner GM, Gavish D, Leopold B, Bolzano K, Weintraub MS, Breslow JL. HMG CoA reductase inhibitors lower LDL cholesterol without reducing Lp(a) levels. Circulation. 1989. 80:1313–1319.15. Marcovina SM, Koschinsky ML, Albers JJ, Skarlatos S. Report of the National Heart, Lung, and Blood Institute Workshop on Lipoprotein (a) and Cardiovascular Disease: recent advances and future directions. Clin Chem. 2003. 49:1785–1796.16. Maher VM, Brown BG, Marcovina SM, Hillger LA, Zhao XQ, Albers JJ. Effects of lowering elevated LDL cholesterol on the cardiovascular risk of lipoprotein (a). JAMA. 1995. 274:1771–1774.17. Insull W Jr, McGovern ME, Schrott H, et al. Efficacy of extended-release niacin with lovastatin for hypercholesterolemia: assessing all reasonable doses with innovative surface graph analysis. Arch Intern Med. 2004. 164:1121–1127.18. Merki E, Graham MJ, Mullick AE, et al. Antisense oligonucleotide directed to human apolipoprotein B-100 reduces lipoprotein (a) levels and oxidized phospholipids on human apolipoprotein B-100 particles in lipoprotein (a) transgenic mice. Circulation. 2008. 118:743–753.19. Kastelein JJ, Wedel MK, Baker BF, et al. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation. 2006. 114:1729–1735.20. Hearn JA, Donohue BC, Báalbaki H, et al. Usefulness of serum lipoprotein (a) as a predictor of restenosis after percutaneous transluminal coronary angioplasty. Am J Cardiol. 1992. 69:736–739.21. Desmarais RL, Sarembock IJ, Ayers CR, Velmon SM, Powers ER, Gimple LW. Elevated serum lipoprotein (a) is a risk factor for clinical recurrence after coronary balloon angioplasty. Circulation. 1995. 91:1403–1409.22. Ribichini F, Steffenino G, Dellavalle A, et al. Plasma lipoprotein (a) is not a predictor for restenosis after elective high-pressure coronary stenting. Circulation. 1998. 98:1172–1177.23. Rhew JY, Jeong MH, Hong YJ, et al. The effects of lipoprotein (a) on coronary stent restenosis. Korean Circ J. 2001. 31:476–483.24. Hoffmann R, Minz GS, Dussaillant GR, et al. Patterns and mechanisms of in-stent restenosis: a serial intravascular ultrasound study. Circulation. 1996. 94:1247–1254.25. Grainger DJ, Kirschenlohr HL, Metcalfe JC, Weissberg PL, Wade DP, Lawn RM. Proliferation of human smooth muscle cells promoted by lipoprotein (a). Science. 1993. 260:1655–1658.26. Morita Y, Himeno H, Yakuwa H, Usui T. Serum lipoprotein (a) level and clinical coronary tenosis progression in patients with myocardial infarction: re-revascularization rate is high in patients with high-Lp(a). Circ J. 2006. 70:156–162.27. Sim DS, Kim JH, Jeong MH. Differences in clinical outcomes between patients with ST-elevation versus non-ST-elevation acute myocardial infarction in Korea. Korean Circ J. 2009. 39:297–303.28. Marcovina SM, Albers JJ, Scanu AM, et al. Use of a reference material proposed by the International Federation of Clinical Chemistry and Laboratory Medicine to evaluate analytical methods for the determination of plasma lipoprotein (a). Clin Chem. 2000. 46:1956–1967.29. Kim CJ, Kwak MH, Kim KM, Ryu WS, Park JT, Ryoo UH. Lipoprotein (a) as an acute phase reactant. Korean J Lipidol. 1996. 6:111–115.30. Heinemann K, Rübig A, Strothmann A, Nahum GG, Heinemann LA. Prevalence and opinions of hormone therapy prior to the Women's Health Initiative: a multinational survey on four continents. J Womens Health (Larchmt). 2008. 17:1151–1166.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Baseline Lipoprotein(a) Levels and Long-Term Cardiovascular Outcomes After Acute Myocardial Infarction
- Off-hour presentation and outcomes for percutaneous coronary intervention in acute myocardial infarction with Killip III–IV
- Comparison of Prognosis According to the Use of Emergency Medical Services in Patients with ST-Segment Elevation Myocardial Infarction
- Differences in the Korea Acute Myocardial Infarction Registry Compared with Western Registries
- Significance of QRS Scoring System in the Acute Myocardial Infarction