Cancer Res Treat.
2004 Oct;36(5):275-286.
Transcription Factors in the Cellular Signaling Network as Prime Targets of Chemopreventive Phytochemicals
- Affiliations
-
- 1National Research Laboratory of Molecular Carcinogenesis and Chemoprevention, College of Pharmacy, Seoul National University Seoul, Korea. surh@plaza.snu.ac.kr
Abstract
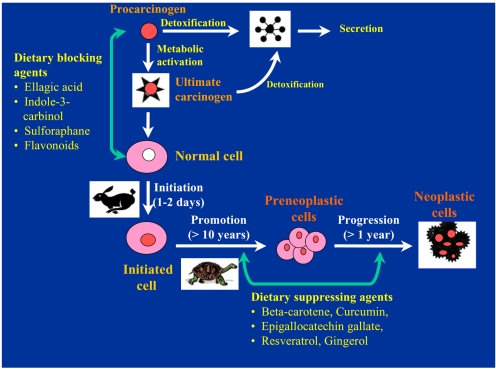
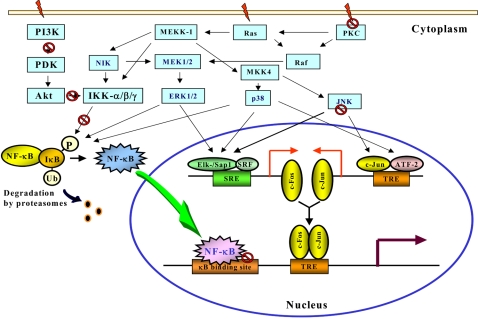
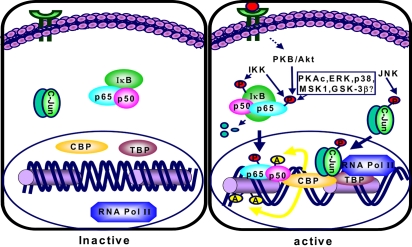
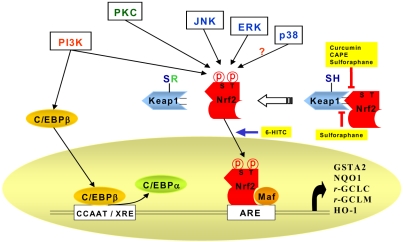
- Accumulating evidence from epidemiologic and laboratory studies support an inverse relationship between a regular consumption of fruits and vegetables and the risk of specific cancers. Numerous phytochemicals derived from edible plants have been reported to possess ability to interfere with a specific stage of carcinogenic process. Multiple mechanisms have been proposed to account for the anti-carcinogenic actions of dietary constituents, but more attention has recently focussed on intracellular signaling cascades as common molecular targets of a wide variety of chemopreventive phytochemicals.
Keyword
MeSH Terms
Figure
Reference
-
1. Surh Y-J. Cancer chemoprevention with dietary phytochemicals. Nature Rev Cancer. 2003; 3:768–780. PMID: 14570043.
Article2. Manson MM. Cancer prevention - the potential for diet to modulate molecular signalling. Trends Mol Med. 2003; 9:11–18. PMID: 12524205.
Article4. Shishodia S, Aggarwal BB. Nuclear factor-κB: a friend or a foe in cancer? Biochem Pharmacol. 2004; 68:1071–1080. PMID: 15313403.
Article5. Milner JA, McDonald SS, Anderson DE, Greenwald P. Molecular targets for nutrients involved with cancer prevention. Nutrition & Cancer. 2001; 41:1–16. PMID: 12094610.
Article6. Gescher A, Pastorino U, Plummer SM, Manson MM. Suppression of tumour development by substances derived from the diet- mechanisms and clinical implications. Br J Clin Pharmacol. 1998; 45:1–12. PMID: 9489587.7. Ashendel CL. Diet, signal transduction and carcinogenesis. J Nutr. 1995; 125:686S–691S. PMID: 7884552.8. Kong AN, Yu R, Hebbar V, Chen C, Owuor E, Hu R, Ee R, Mandlekar S. Signal transduction events elicited by cancer prevention compounds. Mutat Res. 2001; 480-481:231–241. PMID: 11506817.
Article9. Agarwal R. Cell signaling and regulators of cell cycle as molecular targets for prostate cancer prevention by dietary agents. Biochem Pharmacol. 2000; 60:1051–1059. PMID: 11007941.
Article10. Bode AM, Dong Z. Signal transduction pathways: targets for chemoprevention of skin cancer. Lancet Oncol. 2000; 1:181–188. PMID: 11905657.
Article11. Manson MM, et al. Blocking and suppressing mechanisms of chemoprevention by dietary constituents. Toxicol Lett. 2000; 112-113:499–505. PMID: 10720772.
Article12. Owuor ED, Kong AN. Antioxidants and oxidants regulated signal transduction pathways. Biochem Pharmacol. 2002; 64:765–770. PMID: 12213568.
Article13. Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-α-induced cell death. Science. 1996; 274:782–784. PMID: 8864118.14. Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS Jr. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998; 28:1680–1683. PMID: 9733516.
Article15. Visconti R, Cerutti J, Battista S, Fedele M, Trapasso F, Zeki K, Miano MP, de Nigris F, Casalino L, Curcio F, Santoro M, Fusco A. Expression of the neoplastic phenotype by human thyroid carcinoma cell lines requires NF-κB p65 protein expression. Oncogene. 1997; 15:1987–1994. PMID: 9365245.
Article16. Bharti AC, Aggarwal BB. Nuclear factor-κB and cancer: its role in prevention and therapy. Biochem Pharmacol. 2002; 64:883–888. PMID: 12213582.17. Bremner P, Heinrich M. Natural products as targeted modulators of the nuclear factor-κB pathway. J Pharm Pharmacol. 2002; 54:453–472. PMID: 11999122.
Article18. Dong Z, Birrer MJ, Watts RG, Matrisian LM, Colburn NH. Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermal cells. Proc Natl Acad Sci USA. 1994; 91:609–613. PMID: 8290571.
Article19. Dong Z, Lavrovsky V, Colburn NH. Transformation reversion induced in JB6 RT101 cells by AP-1 inhibitors. Carcinogenesis. 1995; 16:749–756. PMID: 7728951.
Article20. Dong Z, Huang C, Brown RE, Ma WY. Inhibition of activator protein 1 activity and neoplastic transformation by aspirin. J Biol Chem. 1997; 272:9962–9970. PMID: 9092536.
Article21. Huang C, Ma WY, Young MR, Colburn N, Dong Z. Shortage of mitogen-activated protein kinase is responsible for resistance to AP-1 transactivation and transformation in mouse JB6 cells. Proc Natl Acad Sci USA. 1998; 95:156–161. PMID: 9419345.
Article22. Huang C, Ma WY, Dong Z. Requirement for phosphatidylinositol 3-kinase in epidermal growth factor-induced AP-1 transactivation and transformation in JB6 P+ cells. Mol Cell Biol. 1996; 16:6427–6435. PMID: 8887671.23. Watts RG, Huang C, Young MR, Li JJ, Dong Z, Pennie WD, Colburn NH. Expression of dominant negative Erk2 inhibits AP-1 transactivation and neoplastic transformation. Oncogene. 1998; 17:3493–3498. PMID: 10030673.
Article24. Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, Howells L. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-κB activation via the NIK/IKK signalling complex. Oncogene. 1999; 18:6013–6020. PMID: 10557090.
Article25. Surh Y-J, Han SS, Keum Y-S, Seo H-J, Lee SS. Inhibitory effects of curcumin and capsaicin on phorbol ester-induced activation of eukaryotic transcription factors, NF-κB and AP-1. Biofactors. 2000; 12:107–112. PMID: 11216470.
Article26. Chun K-S, Keum Y-S, Han SS, Song YS, Kim SH, Surh Y-J. Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extracellular signal-regulated kinase activity and NF-κB activation. Carcinogenesis. 2003; 24:1515–1524. PMID: 12844482.
Article27. Singh S, Aggarwal BB. Activation of transcription factor NF-κB is suppressed by curcumin (diferuloylmethane). J Biol Chem. 1995; 270:24995–25000. PMID: 7559628.
Article28. Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-κB and IκBα kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003; 101:1053–1062. PMID: 12393461.
Article29. Phillip S, Kundu GC. Osteopontin induces nuclear factor κB-mediated promatrix metalloproteinase-2 activation through IκBα/IKK signaling pathways, and curcumin (diferulyolmethane) down-regulates these pathways. J Biol Chem. 2003; 278:14487–14497. PMID: 12473670.30. Nomura M, Ma WY, Huang C, Yang CS, Bowden GT, Miyamoto K, Dong Z. Inhibition of ultraviolet B-induced AP-1 activation by theaflavins from black tea. Mol Carcinog. 2000; 28:148–155. PMID: 10942531.
Article31. Nomura M, Ma W, Chen N, Bode AM, Dong Z. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced NF-κB activation by tea polyphenols, (-)-epigallocatechin gallate and theaflavins. Carcinogenesis. 2000; 21:1885–1890. PMID: 11023547.32. Afaq F, Adhami VM, Ahmad N, Mukhtar H. Inhibition of ultraviolet B-mediated activation of nuclear factor κB in normal human epidermal keratinocytes by green tea Constituent (-)-epigallocatechin-3-gallate. Oncogene. 2003; 22:1035–1044. PMID: 12592390.
Article33. Chung JY, Huang C, Meng X, Dong Z, Yang CS. Inhibition of activator protein 1 activity and cell growth by purified green tea and black tea polyphenols in H-ras-transformed cells: structure-activity relationship and mechanisms involved. Cancer Res. 1999; 59:4610–4617. PMID: 10493515.34. Yang GY, Liao J, Li C, Chung J, Yurkow EJ, Ho CT, Yang CS. Effect of black and green tea polyphenols on c-jun phosphorylation and H2O2 production in transformed and non-transformed human bronchial cell lines: possible mechanisms of cell growth inhibition and apoptosis induction. Carcinogenesis. 2000; 21:2035–2039. PMID: 11062165.35. Kundu JK, Na H-K, Chun K-S, Kim Y-K, Lee SJ, Lee SS, Lee O-S, Sim Y-C, Surh Y-J. Inhibition of phorbol ester- induced COX-2 expression by epigallocatechin gallate in mouse skin and cultured human mammary epithelial cells. J Nutr. 2003; 133:3805S–3810S. PMID: 14608118.36. Nomura M, Kaji A, Ma W, Miyamoto K, Dong Z. Suppression of cell transformation and induction of apoptosis by caffeic acid phenethyl ester. Mol Carcinog. 2001; 31:83–89. PMID: 11429785.
Article37. Pianetti S, Guo S, Kavanagh KT, Sonenshein GE. Green tea polyphenol epigallo catechin-3-gallate inhibits Her-2/neu signaling, proliferation, and transformed phenotype of breast cancer cells. Cancer Res. 2002; 62:652–655. PMID: 11830514.38. Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast cancer cells by inhibiting EGFR-related pathways of signal transduction. J Exp Ther Oncol. 2002; 2:350–359. PMID: 12440226.39. Ahmad N, Gupta S, Mukhtar H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor κB in cancer cells versus normal cells. Arch Biochem Biophys. 2000; 376:338–346. PMID: 10775421.
Article40. Lin JK, Liang YC, Lin-Shiau SY. Cancer chemoprevention by tea polyphenols through mitotic signal transduction blockade. Biochem Pharmacol. 1999; 58:911–915. PMID: 10509743.
Article41. Park OJ, Surh Y-J. Chemopreventive potential of epigallocatechin gallate and genistein: evidence from epidemiological and laboratory studies. Toxicol Lett. 2004; 150:43–56. PMID: 15068824.
Article42. Dampier K, Hudson EA, Howells LM, Manson MM, Walker RA, Gescher A. Differences between human breast cell lines in susceptibility towards growth inhibition by genistein. Br J Cancer. 2001; 85:618–624. PMID: 11506505.
Article43. Tacchini L, Dansi P, Matteucci E, Desiderio MA. Hepatocyte growth factor signal coupling to various transcription factors depends on triggering of Met receptor and protein kinase transducers in human hepatoma cells HepG2. Exp Cell Res. 2000; 256:272–281. PMID: 10739674.
Article44. Wang Y, Zhang X, Lebwohl M, DeLeo V, Wei H. Inhibition of ultraviolet B (UVB)-induced c-fos and c-jun expression in vivo by a tyrosine kinase inhibitor genistein. Carcinogenesis. 1998; 19:649–654. PMID: 9600350.
Article45. Davis JN, Kucuk O, Sarkar FH. Genistein inhibits NF-κB activation in prostate cancer cells. Nutr Cancer. 1999; 35:167–174. PMID: 10693171.46. Li Y, Sarkar FH. Inhibition of nuclear factor κB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res. 2002; 8:2369–2377. PMID: 12114442.47. Gong L, Li Y, Nedeljkovic-Kurepa A, Sarkar FH. Inactivation of NF-κB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene. 2003; 22:4702–4709. PMID: 12879015.
Article48. Chen CC, Sun YT, Chen JJ, Chiu KT. TNF-alpha-induced cyclooxygenase-2 expression in human lung epithelial cells: involvement of the phospholipase C-γ2, protein kinase C-α, tyrosine kinase, NF-κB-inducing kinase, and I-κB kinase 1/2 pathway. J Immunol. 2000; 165:2719–2728. PMID: 10946303.49. Nasuhara Y, Adcock IM, Catley M, Barnes PJ, Newton R. Differential IkappaB kinase activation and IκBα degradation by interleukin-1β and tumor necrosis factor-α in human U937 monocytic cells. Evidence for additional regulatory steps in κB-dependent transcription. J Biol Chem. 1999; 274:19965–19972. PMID: 10391945.50. Subbaramaiah K, Chung WJ, Michaluart P, Telang N, Tanabe T, Inoue H, Jang M, Pezzuto JM, Dannenberg AJ. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J Biol Chem. 1998; 273:21875–21882. PMID: 9705326.
Article51. Subbaramaiah K, Michaluart P, Chung WJ, Tanabe T, Telang N, Dannenberg AJ. Resveratrol inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Ann N Y Acad Sci. 1999; 889:214–223. PMID: 10668496.
Article52. Mouria M, Gukovskaya AS, Jung Y, Buechler P, Hines OJ, Reber HA, Pandol SJ. Food-derived polyphenols inhibit pancreatic cancer growth through mitochondrial cytochrome c release and apoptosis. Int J Cancer. 2002; 98:761–769. PMID: 11920648.
Article53. Banerjee S, Bueso-ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kB, cyclooxygenase-2, and matrix metalloprotease 9. Cancer Res. 2002; 62:4945–4954. PMID: 12208745.54. Narayana BA, Narayana NK, Re GG, Nixon DW. Differential expression of genes induced by resveratrol in LNCaP cells: p53-mediated molecular targets. Int J Cancer. 2003; 104:204–212. PMID: 12569576.55. She QB, Bode AM, Ma WY, Chen NY, Dong Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-mediated protein kinase and p38 kinase. Cancer Res. 2001; 61:1604–1610. PMID: 11245472.56. Yu R, Hebbar V, Kim DW, Mandlekar S, Pezzuto JM, Kong AN. Resveratrol inhibits phorbol ester and UV-induced activator protein 1 activation by interfering with mitogen-activated protein kinase pathways. Mol Pharmacol. 2001; 60:217–224. PMID: 11408617.
Article57. Adhami VM, Afaq F, Ahmad N. Suppression of ultraviolet B exposure-mediated activation of NF-κB in normal human keratinocytes by resveratrol. Neoplasia. 2003; 5:74–82. PMID: 12659672.
Article58. Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factor NF-κB, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000; 164:6509–6519. PMID: 10843709.59. Holmes-McNary M, Baldwin AS Jr. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IκB kinase. Cancer Res. 2000; 60:3477–3483. PMID: 10910059.60. Lee JS, Surh Y-J. Cancer Lett. Nrf2 as a novel molecular target for chemoprevention. Cancer. Lett in press.61. Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997; 236:313–322. PMID: 9240432.
Article62. Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive ditholedithiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem. 2003; 278:8135–8145. PMID: 12506115.63. Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice.[comment]. Proc Natl Acad Sci USA. 2001; 98:3410–3415. PMID: 11248092.64. Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci USA. 2001; 98:4611–4616. PMID: 11287661.
Article65. McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, Wolf CR, Cavin C, Hayes JD. The Cap'n Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001; 61:3299–3307. PMID: 11309284.66. Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRE2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002; 26:175–182. PMID: 11804867.67. Ramos-Gomez M, Dolan PM, Itoh K, Yamamoto M, Kensler TW. Interactive effects of nrf2 genotype and oltipraz on benzo[ a]pyrene-DNA adducts and tumor yield in mice. Carcinogenesis. 2003; 24:461–467. PMID: 12663505.68. Kwak MK, Egner PA, Dolan PM, Ramos-Gomez M, Groopman JD, Itoh K, Yamamoto M, Kensler TW. Role of phase 2 enzyme induction in chemoprotection by dithiolethiones. Mutat Res. 2001; 480-481:305–315. PMID: 11506823.
Article69. Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999; 274:26071–26078. PMID: 10473555.
Article70. Chan JY, Kwong M. Impaired expression of glutathione synthetic enzyme genes in mice with targeted deletion of the Nrf2 basic-leucine zipper protein. Biochim Biophys Acta. 2000; 1517:19–26. PMID: 11118612.
Article71. Nguyen T, Huang HC, Pickett CB. Transcriptional regulation of the antioxidant response element. Activation by Nrf2 and repression by MafK. J Biol Chem. 2000; 275:15466–15473. PMID: 10747902.72. Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999; 13:76–86. PMID: 9887101.
Article73. Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talaly P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci USA. 2002; 99:11908–11913. PMID: 12193649.
Article74. Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA. 2002; 99:11908–11913. PMID: 12193649.
Article75. Wolf CR. Chemoprevention: increased potential to bear fruit. Proc Natl Acad Sci USA. 2001; 98:2941–2943. PMID: 11248007.
Article76. Yu R, Mandlekar S, Lei W, Fahl WE, Tan TH, Kong AT. p38 mitogen-activated protein kinase negatively regulates the induction of phase II drug-metabolizing enzymes that detoxify carcinogens. J Biol Chem. 2000; 275:2322–2327. PMID: 10644681.
Article77. Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidantresponsive element. Biochem J. 2003; 371:887–895. PMID: 12570874.
Article78. Kang KW, Lee SJ, Park JW, Kim SG. Phosphatidylinositol 3-kinase regulates nuclear translocation of NF-E2-related factor 2 through actin rearrangement in response to oxidative stress. Mol Pharmacol. 2002; 62:1001–1010. PMID: 12391262.
Article79. Lee JM, Hanson JM, Chu WA, Johnson JA. Phosphatidylinositol 3-kinase, not extracellular signal-regulated kinase, regulates activation of the antioxidant-responsive element in IMR-32 human neuroblastoma cells. J Biol Chem. 2001; 276:20011–20016. PMID: 11274155.
Article80. Kang KW, Choi SH, Kim SG. Peroxynitrite activates NF-E2-related factor 2/antioxidant response element through the pathway of phosphatidylinositol 3-kinase: the role of nitric oxide synthase in rat glutathione S-transferase A2 induction. Nitric Oxide. 2002; 7:244–253. PMID: 12446173.
Article81. Nakaso N, Yano H, Fukuhara Y, Takeshima T, Wada-Isoe K, Nakashima K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 2003; 546:181–184. PMID: 12832036.
Article82. Kang KW, Park EY, Kim SG. Activation of CCAAT/enhancer-binding protein beta by 2'-amino-3'-methoxyflavone (PD 98059) leads to the induction of glutathione S-transferase A2. Carcinogenesis. 2003; 24:475–482. PMID: 12663507.83. Kang KW, Cho IJ, Lee CH, Kim SG. Essential role of phosphatidylinositol 3-kinase-dependent CCAAT/enhancer binding protein beta activation in the induction of glutathione S-transferase by oltipraz. J Natl Cancer Inst. 2003; 95:53–66. PMID: 12509401.84. Yu R, Lei W, Mandlekar S, Weber MJ, Der CJ, Wu J, Kong AT. Role of a mitogen-activated protein kinase pathway in the induction of phase II detoxifying enzymes by chemicals. J Biol Chem. 1999; 274:27545–27552. PMID: 10488090.
Article85. Yu R, Chen C, Mo YY, Hebbar V, Owuor ED, Tan TH, Kong AN. Activation of mitogen-activated protein kinase pathways induces antioxidant response element-mediated gene expression via a Nrf2-dependent mechanism. J Biol Chem. 2000; 275:39907–39913. PMID: 10986282.
Article86. Katoh Y, Itoh K, Yoshida E, Miyagishi M, Fukamizu A, Yamamoto M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells. 2001; 6:857–868. PMID: 11683914.
Article87. Zhu M, Fahl WE. Functional characterization of transcription regulators that interact with the electrophile response element. Biochem Biophys Res Commun. 2001; 289:212–219. PMID: 11708801.
Article88. Shen G, Hebbar V, Nair S, Xu C, Li W, Lin W, Keum YS, Han J, Gallo MA, Kong AN. Regulation of Nrf2 transactivation domain activity. The differential effects of mitogen- activated protein kinase cascades and synergistic stimulatory effect of Raf and CREB-binding protein. J Biol Chem. 2004; 279:23052–23060. PMID: 15020583.89. Huang MT, Newmark HL, Frenkel K. Inhibitory effects of curcumin on tumorigenesis in mice. J Cell Biochem Suppl. 1997; 27:26–34. PMID: 9591190.
Article90. Chun K-S, Park KK, Lee J, Kang M, Surh Y-J. Inhibition of mouse skin tumor promotion by anti-inflammatory diarylheptanoids derived from Alpinia oxyphylla Miquel (Zingiberaceae). Oncol Res. 2002; 13:37–45. PMID: 12201673.
Article91. Chun K-S, Keum Y-S, Han SS, Song YS, Kim SH, Surh Y-J. Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extracellular signal-regulated kinase activity and NF-κB activation. Carcinogenesis. 2003; 24:1515–1524. PMID: 12844482.
Article92. Azuine MA, Bhide SV. Chemopreventive effect of turmeric against stomach and skin tumors induced by chemical carcinogens in Swiss mice. Nutr Cancer. 1992; 17:77–83. PMID: 1574446.
Article93. Huang MT, Lou YR, Ma W, Newmark HL, Reuhl KR, Conney AH. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994; 54:5841–5847. PMID: 7954412.94. Rao CV, Simi B, Reddy BS. Inhibition by dietary curcumin of azoxymethane-induced ornithine decarboxylase, tyrosine protein kinase, arachidonic acid metabolism and aberrant crypt foci formation in the rat colon. Carcinogenesis. 1993; 14:2219–2225. PMID: 8242846.
Article95. Michaluart P, Masferrer JL, Carothers AM, Subbaramaiah K, Zweifel BS, Koboldt C, Mestre JR, Grunberger D, Sacks PG, Tanabe T, Dannenberg AJ. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res. 1999; 59:2347–2352. PMID: 10344742.96. Rao CV, Desai D, Simi B, Kulkarni N, Amin S, Reddy BS. Inhibitory effect of caffeic acid esters on azoxymethane-induced biochemical changes and aberrant crypt foci formation in rat colon. Cancer Res. 1993; 53:4182–4188. PMID: 8364913.97. Huang MT, Ma W, Yen P, Xie JG, Han J, Frenkel K, Grunberger D, Conney AH. Inhibitory effects of caffeic acid phenethyl ester (CAPE) on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion in mouse skin and the synthesis of DNA, RNA and protein in HeLa cells. Carcinogenesis. 1996; 17:761–765. PMID: 8625488.
Article98. Dickinson DA, Iles KE, Zhang H, Blank V, Forman HJ. Curcumin alters EpRE and AP-1 binding complexes and elevates glutamate-cysteine ligase gene expression. FASEB J. 2003; 17:473–475. PMID: 12514113.
Article99. Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992; 89:2399–2403. PMID: 1549603.
Article100. Ye L, Zhang Y. Total intracellular accumulation levels of dietary isothiocyanates determine their activity in elevation of cellular glutathione and induction of Phase 2 detoxification enzymes. Carcinogenesis. 2001; 22:1987–1992. PMID: 11751429.
Article101. Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci USA. 1997; 94:10367–10372. PMID: 9294217.
Article102. Kong AN, Owuor E, Yu R, Hebbar V, Chen C, Hu R, Mandlekar S. Induction of xenobiotic enzymes by the MAP kinase pathway and the antioxidant or electrophile response element (ARE/EpRE). Drug Metab Rev. 2001; 33:255–271. PMID: 11768769.
Article103. Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002; 62:5196–5203. PMID: 12234984.104. Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene- induced stomach tumors. Proc Natl Acad Sci USA. 2002; 99:7610–7615. PMID: 12032331.105. Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci USA. 1994; 91:3147–3150. PMID: 8159717.
Article106. Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000; 2:2287–2291. PMID: 11133820.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation of Cellular Antiviral Signaling by Modifications of Ubiquitin and Ubiquitin-like Molecules
- Signal Transduction Pathways in Colorectal Cancer Carcinogenesis and Metastasis
- Survive or thrive: tradeoff strategy for cellular senescence
- Molecular mechanisms of long bone growth and chondrocyte regulation: A narrative review
- The use of intra-cellular signaling pathways in anesthesiology and pain medicine field